DISCUSSION POINTS
• The propagation system
..– Research protocols
..– Back to the basics
..– Controlling variables
..– Supplemental lighting
..– Developing a propagation system
• Results of propagation experimentation
..– Cornus kousa ‘Scarlet Fire’
..– Corylus avellana – various selections
..– Vaccinium macrocarpon ‘Haines’
..– Ilex x? & Ilex crenata ‘Beehive’
• Moving toward the future
WHAT ARE “CHALLENGING” PLANTS
Experimental design: Using a systematic approach
Propagation variables
Limiting the variables
Auxins
Control of variable combinations
RESEARCH PROTOCOLS & BACK TO THE BASICS
What are challenging plants?
• I describe challenging plants as plants that others have had problems propagating
• How to review scientific literature
..– Look for hints at propagation history or propagation of related plants
..– Always check experimental design to be sure there are adequate controls built into the experiment
..– Without proper experimental controls, information is nearly useless
• Recognize that timing is very important
..– Plants are more successfully propagated at certain times of the year
..– BUT, don’t necessarily eliminate other times of the year if it’s possible to change experimental designs and protocols
EXPERIMENTAL DESIGN: USE A SYSTEMATIC APPROACH
•• Since one never knows if a new system will really work
..– Limit your exposure by starting with a manageable number of plants
• There is the potential for crop and financial losses
• Make sure you have enough cuttings to see a difference
• Have an appropriate “control” group of plants*
• Decide if the experiment is a “proof of concept”, a comparison against the “present best treatment” or a combination of the two.
..– Allow space for sequential propagation cycles
• Optimal timing can be measured in days
• Success can vary by the hour of the day one collects cuttings
• Rooting success for tissue cultured shoots will vary by size and maturity
..– Remember that plant propagation is nearly as much an art as a science
PROPAGATION VARIABLES
• Air & media temperatures: turgidity & transpiration
• Auxins: which one, what carrier, how to apply
• Fertility: how much & how long after root initiation
• Humidity
..– Condensing (wet leaves as with misting)
..– Non-condensing (dry leaves as with true fog)
• Light: quality, intensity, duration
• Media: texture, moisture content, cation exchange capacity (CEC)
• Timing: based on the physiological stage of growth
• Types of cells & trays
LIMITING THE VARIABLES
• Focus: avoid looking for a bunch of answers at the same time
• Keep the number of test plants high to see differences
• Temperature & humidity
..– Ventilation for heat will reduce both temperature and humidity
..– Condensing humidity can cause foliar and root disease problems
..– Condensing humidity can leach foliar nutrients
..– Non-condensing humidity can resolve many of the temperature/humidity issues but it can be costly
• Media & containers
..– Use a porous medium to limit excess water issues
..– Use a deep cell or tray to maximize useful media
• When one removes a competing variable, rooting success increases
AUXINS
• IAA
..– Indole-3-acetic acid
..– Naturally occurring plant hormone
• IBA
– Indole-3-butyric acid
..– Naturally occurring plant hormone
..– Available commercially as Hortus IBA Water Soluble Salts the water soluble form of the IBA plant rooting hormone
• NAA
..– 1-naphthalene-acetic acid
..– A synthetic rooting hormone
• Auxin movement
..– Foliar applied auxins move more readily with more light (2, 4-D)
..– Basal applied auxins more through the xylem in the transpiration stream
CONTROL OF VARIABLE COMBINATIONS
• Temperature & humidity
..– Ventilation for heat will reduce both temperature and humidity
..– Supplemental condensing humidity can cause foliar and root disease problems
..– Supplemental condensing humidity can cause leaching of foliar nutrients
..– Non-condensing humidity can resolve many of the temperature/humidity issues but it can be costly
• Media & containers
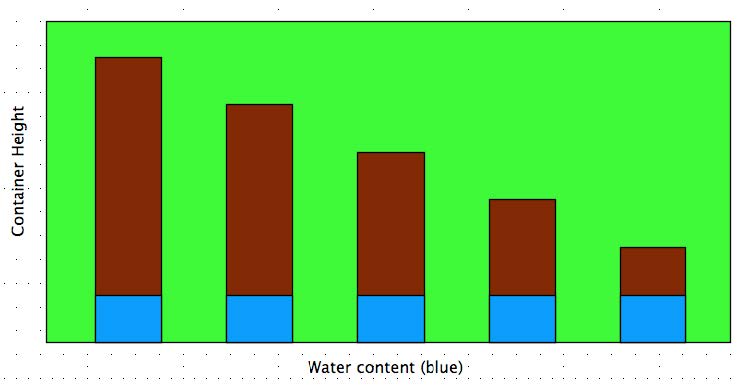
..– Perched water table (see below). Accumulation of groundwater located above a water table in an unsaturated zone.
..– When using the same medium, success is largely controlled by the height of the container
PERCHED WATER TABLE
SUPPLEMENTAL LIGHTING
Photosynthesis: we don’t see what plants see
A system that can work in the real world
DEVELOPING A PROPAGATION SYSTEM
SUPPLEMENTAL LIGHTING
• Has the ability to can change the season
• LED’s (light emitting diodes) produce light by electroluminescence
..– They generate less heat and use less electricity than conventional lights
• Light quality can be fine tuned by use of different wavelength generating LED’s
..– Most LED lights have lights for vegetative growth and a second set of lights for flowering built into a single light-set
..– Propagation only needs wavelengths suitable for vegetative growth
• Cost effective
..– LED lighting has a higher initial investment but has longer life
..– From my experience, the system has enhanced rooting
PHOTOSYNTHESIS: WE DON’T SEE WHAT PLANTS SEE
• Plant chlorophylls efficiently harvest blue and red light with peak efficiency at about 440 and 640 nm
• They don’t capture light between 500-575 nm
• Plants reflect light they can’t capture (image photosynthesis)
 

THE SYSTEM
• Reflector Led Grow Light 576w (192-3w LED's) @$304
• Heavy duty light timer @$15
• Grow Tent (4' 9'' x 4' 9'' x6' 7'') @$136
• Ductwork, ductstat, thermostat & rheostat @$134
• 15 amp heavy duty power station/surge suppresser @$28
• Propagating heat mats w thermostat & tall domes @$326
• 50 deep cell plug trays (1.94” x 4.5”) @$121
• D.B. Smith Contractor Sprayer (mist) @$26
• Honeywell 1 Gal. Cool Mist Humidifier (fog) @$58
• Plastic pallet bench @$11
• Total system cost = @$1159
A SYSTEM THAT CAN WORK IN THE REAL WORLD
• Set goals when evaluating propagation system changes
• Supplemental lighting can enhance rooting
..– Lighting can effectively change and/or extend the season
• Reduction of environmental impact
..– Water use is limited by the use of non-condensing humidity
..– High humidity levels also reduces loss of water through transpiration
• Worker safety
..– Spray applied auxin offers a method of minimizing worker contact with auxins
• Cost effective
..– LED lighting has a higher initial investment but has longer life
..– LED lighting has a lower operational cost
Cornus kousa ‘Scarlet Fire’
• K-IBA
..– Available commercially as Hortus IBA Water Soluble Salts
RESULTS OF PROPAGATION EXPERIMENTS
Cornus kousa ‘Scarlet Fire’
• Dogwoods
..– Production of tissue cultured shoots has worked very well
..– Producing roots on those shoots has not been successful in TC
..– An experiment was initiated that looked at hormone rates and frequency of application of foliar applied auxins
• Optimal treatments
..– Tray size: 50 cell, deep tray (4.5” deep)
..– Auxin: 1 foliar application to drip of K-IBA (Hortus IBA Water Soluble Salts) at 350-400 ppm
..– Fertilization: complete at 70 to 75 ppm when rooting is initiated
..– Shading for the first 7 days didn’t make a difference
• Results
..– Typically between 95 and 100% success
Cornus kousa ‘Scarlet Fire’ TC shoots
 
Newly stuck Cornus kousa ‘Scarlet Fire’

CORNUS KOUSA ‘SCARLET FIRE’ PROPAGATION PROCEDURE
• Keep the shoots in the covered agar medium for 2 days to harden
• Day 1: Stick the shoots and gently water them in so they are in good contact with the medium.
Be careful not to dislodge the shoots.
..– Operate the LED vegetative grow lights for 14 hour days
• Day 2: Apply the K-IBA (Hortus IBA Water Soluble Salts) at 350 to 400 ppm as a foliar spray that fully wets the leaves
..– Mist the inside of the domes, supplementing the humidifier
..– Check the shoots later in the day and mist the inside of the dome as well as the leaves again if they are dry
• Day 3 onward: Check the humidity and mist 2 to 3 times a day as necessary (it’s normal early in the propagation period).
PROPAGATION PROCEDURE
• Day 7: Start checking for root initiation. As soon as the first roots appear, start fertilization at 75 ppm-N.
Continue misting until top growth is established.
• Day 12: Top growth should be initiating
• Day 20: Start reducing humidity by removing the domes. Continue growing plants until the desire size is reached.
..– New growth is fairly active at about 22 or 23 days
..– Plants may be as much as 4 to 6 inches tall in 60 days
• Notes:
..– There seem to be some variability in success based on the maturity of the tissue cultured shoots.
..– Use of NAA was not successful
Cornus kousa ‘Scarlet Fire’ at 60 days

CORYLUS AVELLANA
• Hazelnuts: a tough nut to crack
..– I used the same general system as for dogwoods
..– Cuttings were traditional stem cuttings, usually from immature suckers
..– Rooting was normally in 38 cell deep trays (5”deep)
..– A combination of IBA and NAA seemed to work best
..– Mid-September dates seemed to offer the most success
• Cuttings were taken from August through November and in late-January
..– Excess callus was consistently an issue
..– The rooted stick
..– Success with hazelnuts was really not successful with the best treatments achieving around 20% to 50% rooting
..– There was a high degree of varietal variability in success rate
..– Once rooted, they grow exceptionally well
CALLUS AND THE ROOTED STICK

callus

rooted stick
CORYLUS AVELLANA
 
VACCINIUM MACROCARPON ‘HAINES’
• Rutgers researchers have developed a new, hardier variety of cranberry that is able to withstand disease and has a larger round berry with a more even color than other varieties
• It’s focused on the Craisins® market

PROPAGATION OF THE ‘HAINES’ VARIETY
• Production of tissue cultured shoots with roots has been successful
• Traditional multiplication by softwood cuttings had poor results
• An experiment was set up to evaluate hormone rates
• Ultimately, rooting without hormones was the best treatment as is traditionally done
• The problems were associated with short cells and a perched water table causing cuttings to be stuck into saturated zones
ADDITIONAL NOTES AND UNINTENDED CONSEQUENCES
• Cuttings rooted above 95% without IBA in deep cell trays
• While no hormone was ultimately the preferred treatment, cuttings rooted more aggressively with the use of IBA at 200 to 400 ppm as a spray application
• The unintended consequence is that top growth was effectively inhibited when using foliar applied IBA
..– The higher the rate of IBA applied, the longer it took for top growth to restart
• I was unable to experiment with basal applied IBA as a treatment due to time constraints
..– Economically, it would probably not be cost effective anyway
ILEX X?

Ilex x? - Surprises happen
• Over 30 years ago while walking through the Rutgers Gardens with Dr. Elwin Orton, I came across a holly tree that had no leaf miner and a glossy ovate leaf with spines
• I asked Dr. Elwin Orton what variety it was and he indicated, colorfully, that years earlier the USDA had initiated a variety evaluation trial and then lost the plot plan
• I took quite a few cuttings and rooted a few using traditional methods of an IBA talc basal dip
• I continue to like the plant so I took cuttings in mid-March in an effort to root some to take with me into my retirement
• Out of 10 cuttings, all rooted.
ILEX CRENATA ‘BEEHIVE’
• This is a plant that Dr. Elwin Orton selected quite a few years ago
• It’s also another that I wanted to have in my retirement landscape
• I took cuttings in mid-March
• Of the 30 cuttings taken, 29 rooted

MOVING TOWARD THE FUTURE
• We are looking at more of the same
..– More regulation
..– Less labor
• That results in the need for
..– More intensive agricultural operations
..– Less employee exposure to risks
..– More mechanization
• This system has lower operational cost
• The system can produce a lot of plants in a small space using LED lighting, non-condensing humidity and bottom heat
• Existing propagation space can be integrated as a step-down system
CORNUS KOUSA 'SCARLET FIRE'

Presentation closing |