
Glass Blowing for Vacuum Devices
Magnesium Photoelectric Cell
When electromagnetic radiation falls on a metal surface, electrons can be released.
Electron emission only occurs if the frequency of the radiation is above a value called
the threshold frequency. The threshold frequency is dependent upon the type of material.
Wave theory does not explain the photoelectric effect. Instead, it is necessary to
consider the radiation as particles called photons. The energy of each photon is related
to the frequency by E = hf where E is the energy in joules (J), h is Planck's constant
(6.626x10-34Js) and f is the frequency in Hz. Joules can be converted to electron volts
by dividing by 1.6x10-19.
Each metallic element has a fixed value called its work function, which is usually expressed
in electron volts (eV). If photons striking the material have energies greater than its work
function, electrons will be released. This means that the lower the work function a material
has, the lower the frequency and hence the longer the wavelength of electromagnetic radiation
that will cause electron emission. Some elements such as caesium (1.95eV) and sodium (2.36eV)
exhibit photoelectric emission with visible light, but the threshold of most metals is in the
ultraviolet.
A photoelectric cell consists of a very clean surface of a low work function material in
a vacuum. One connection is made to this surface, which is called the photocathode. A second
electrode (the anode) is positioned in front of the photocathode. A voltage is applied from
anode to cathode, so that electrons which are emitted are electrostatically attracted to
the anode. The resulting current is then measured and is proportional to the intensity as
long as the wavelength of the light is constant.
I first tried making a photoelectric cell using sodium obtained from a failed low pressure
sodium lamp. I coiled tungsten wire around the sodium so that it could be evaporated. The
problem was that sodium is so reactive that by the time I had assembled the bulb and fitted
it to the pump, it had all converted to sodium hydroxide due to moisture in the air. The
lowest work function metals are mostly in the left hand column of the periodic table and are
therefore very reactive making them difficult and dangerous to handle.
Magnesium has a work function of about 3.66eV which is less than most metals. For
photoelectric emission to occur, the photons would require energies of 3.66 x
1.6x10-19 = 5.86x10-19J. The threshold frequency is therefore
5.86x10-19 / 6.626x10-34 = 8.84x1014Hz. The threshold
wavelength is therefore λ = c/f = 3x108 / 8.84x1014 = 339nm.
This is just beyond violet, in the soft ultraviolet part of the spectrum.
 I used a construction very similar to
'Filament Lamp B'.
The only difference in the envelope is that I added a valve base pin in the side wall of
the bulb. I did this by pre-beading the pin with glass. I blew out a small area on the
side of the bulb using a sharp flame and fused the beaded pin into it. I found that re-heating
the entire bulb again with the pin in it until it starts to collapse and then blowing it out
again gives a stress free result. This has to be done before the pump tube is attached.
I used a construction very similar to
'Filament Lamp B'.
The only difference in the envelope is that I added a valve base pin in the side wall of
the bulb. I did this by pre-beading the pin with glass. I blew out a small area on the
side of the bulb using a sharp flame and fused the beaded pin into it. I found that re-heating
the entire bulb again with the pin in it until it starts to collapse and then blowing it out
again gives a stress free result. This has to be done before the pump tube is attached.
 This is the stem, which was made in the same way as that of 'Filament Lamp B'. I spot
welded a rectangle of steel sheet to one of the nickel/iron wires using the
home-made
spot welder. I crimped a thin strip of magnesium between the second lead-in wire and the
end of the steel sheet. Passing current through the magnesium in a vacuum will cause it to
evaporate on to the inside surface of the bulb and make electrical contact to the valve
pin.
This is the stem, which was made in the same way as that of 'Filament Lamp B'. I spot
welded a rectangle of steel sheet to one of the nickel/iron wires using the
home-made
spot welder. I crimped a thin strip of magnesium between the second lead-in wire and the
end of the steel sheet. Passing current through the magnesium in a vacuum will cause it to
evaporate on to the inside surface of the bulb and make electrical contact to the valve
pin.
 This is the completed cell. Getting the stem to fuse to the bulb tube with the magnesium
ribbon correctly aligned with the pin was quite difficult.
This is the completed cell. Getting the stem to fuse to the bulb tube with the magnesium
ribbon correctly aligned with the pin was quite difficult.
The purpose of the metal plate is to act as a shield so that magnesium does not coat the
inside of the bulb opposite the pin. This provides a window for light to enter.
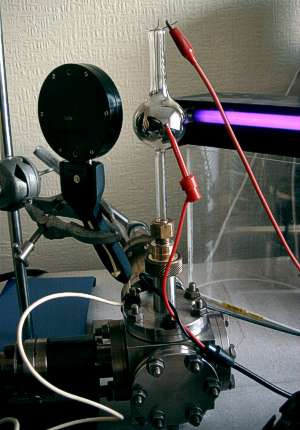 Here, the cell is mounted on the vacuum system and the magnesium has been evaporated. I
had trouble getting it to evaporate. I made the magnesium ribbon too wide. As a result,
I had to pass such a heavy current that the lead-in wires also started to evaporate.
Surprisingly, the glass-metal seals did not fail catastrophically and a working cell
was achieved.
Here, the cell is mounted on the vacuum system and the magnesium has been evaporated. I
had trouble getting it to evaporate. I made the magnesium ribbon too wide. As a result,
I had to pass such a heavy current that the lead-in wires also started to evaporate.
Surprisingly, the glass-metal seals did not fail catastrophically and a working cell
was achieved.
The black disc on the left is a light meter. The purple light on the right is an
ultraviolet lamp intended for checking bank notes.
The voltage source was a Weir regulated bench power supply. The current was measured using
a Cooknell Electronics CP13 pico-ammeter. The luminous flux was measured using a Diffusion
Systems photometer. The photometer was calibrated for a tungsten light source and is therefore
unlikely to be accurate for a UV source, but will give meaningful relative measurements.
Luminous flux values are given in lux which is lumens per square meter.
The characteristics were measured at an indicated luminous flux of 44 lux and 88 lux.
The results are shown in the graph below. As the voltage is increased, the current saturates
at around 30V. This corresponds to all of the electrons emitted by the cathode being
collected by the anode. The saturated current at 44 lux was 8.3nA and at 88 lux it was 16.5nA.
This indicates that the current had very good proportionality to the luminous flux.

The cell had some sensitivity to a tungsten filament lamp. At 76 lux, the saturated
current was 9.1pA compared to a dark current of 4.2pA.
The above measurements were taken with the cell still on the pump. After the cell was
sealed-off and detached from the pump, the sensitivity decreased dramatically. It is
possible that overheating the lead-in wires damaged the glass-metal seals. It is difficult
to tell because of metal deposited on the end of the stem.
Note added October 2009: The loss of sensitivity after sealing-off was almost certainly
due to poor vacuum. The glass had not been degassed.
 I used a construction very similar to
'Filament Lamp B'.
The only difference in the envelope is that I added a valve base pin in the side wall of
the bulb. I did this by pre-beading the pin with glass. I blew out a small area on the
side of the bulb using a sharp flame and fused the beaded pin into it. I found that re-heating
the entire bulb again with the pin in it until it starts to collapse and then blowing it out
again gives a stress free result. This has to be done before the pump tube is attached.
I used a construction very similar to
'Filament Lamp B'.
The only difference in the envelope is that I added a valve base pin in the side wall of
the bulb. I did this by pre-beading the pin with glass. I blew out a small area on the
side of the bulb using a sharp flame and fused the beaded pin into it. I found that re-heating
the entire bulb again with the pin in it until it starts to collapse and then blowing it out
again gives a stress free result. This has to be done before the pump tube is attached.
 This is the stem, which was made in the same way as that of 'Filament Lamp B'. I spot
welded a rectangle of steel sheet to one of the nickel/iron wires using the
This is the stem, which was made in the same way as that of 'Filament Lamp B'. I spot
welded a rectangle of steel sheet to one of the nickel/iron wires using the
 This is the completed cell. Getting the stem to fuse to the bulb tube with the magnesium
ribbon correctly aligned with the pin was quite difficult.
This is the completed cell. Getting the stem to fuse to the bulb tube with the magnesium
ribbon correctly aligned with the pin was quite difficult. Here, the cell is mounted on the vacuum system and the magnesium has been evaporated. I
had trouble getting it to evaporate. I made the magnesium ribbon too wide. As a result,
I had to pass such a heavy current that the lead-in wires also started to evaporate.
Surprisingly, the glass-metal seals did not fail catastrophically and a working cell
was achieved.
Here, the cell is mounted on the vacuum system and the magnesium has been evaporated. I
had trouble getting it to evaporate. I made the magnesium ribbon too wide. As a result,
I had to pass such a heavy current that the lead-in wires also started to evaporate.
Surprisingly, the glass-metal seals did not fail catastrophically and a working cell
was achieved.