
With no assessment of health risks to infants, federal regulators have approved a hormone-disrupting pesticide, triclosan, for use in 140 different types of consumer products including liquid hand soap, toothpaste, undergarments and children's toys. This exposure has been allowed despite the fact that the chemical ends up in mothers' breast milk and poses potential toxicity to fetal and childhood development. 
In addition to these risks, Environmental Working Group (EWG) finds no evidence that triclosan's widespread use in liquid hand soap and other products gives consumers the germ-killing benefits they are promised. The American Medical Association, a Food and Drug Administration advisory committee, and dozens of academic researchers have determined that antimicrobial soap does not work any better than plain soap and water at preventing the spread of infections or reducing bacteria on the skin.
As required by law, the Environmental Protection Agency is now reviewing health and safety data for triclosan. This is a critical process that could lead to the stringent health and environmental protections needed to reduce exposure to this toxic antimicrobial agent. However, EPA's draft risk assessment of triclosan gives cause for concern: Plagued with data gaps and inconsistencies, the assessment was crafted to support the status quo.
EPA has approved triclosan for use in 20 pesticide formulations applied to consumer products from credit cards and countertops to baby bibs and blankets. In a callous and unjustified abuse of federal pesticide law, EPA failed to consider the safety of babies' and children's exposure to triclosan in breast milk, mattresses, sleepers, blankets, bibs, toys, house dust, diaper cream, and other potential sources when approving these uses.
Triclosan persists in the environment, breaks down into substances highly toxic to wildlife, pollutes the human body, and poses health risks that are barely studied and poorly understood. Because triclosan has been proven ineffective, and EPA has failed to assess its safety for children, we recommend:
- A ban on triclosan in personal care products and any other products used at home, in line with the conclusion of the American Medical Association that common antimicrobials for which resistance has been demonstrated should "be discontinued in consumer products unless data emerge that conclusively show that such resistance has no effect on public health and that such products are effective at preventing infection."
- For remaining non-consumer uses, EPA must fully assess the safety of triclosan and its breakdown products for the fetus, infant, child, and other vulnerable populations.
- Consumers should avoid the use of triclosan-laden products whenever possible.
- Manufacturers should curtail their use of this toxic, persistent chemical in consumer products, voluntarily in advance of mandatory restrictions.
Triclosan in consumer products leads to widespread
pollution in people and the environment
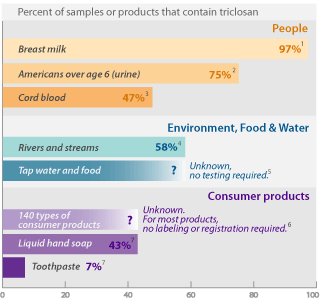
Notes and references
1 Testing sponsored by triclosan manufacturer Ciba found the pesticide in 60 of 62 breast milk samples from mothers in San Jose, CA and Austin, TX (Dayan 2007).
2 Centers for Disease Control and Prevention (CDC) detected triclosan in 75% of 2,517 people 6 years of age and higher, in tests of the chemical in urine samples (Calafat 2008).
3 Triclosan was detected in 8 of 17 cord blood samples in a study conducted in the Netherlands (TNO 2005).
4 The U.S. Geological Survey (USGS) detected triclosan in 58% of 85 rivers and streams tested in 30 states (Kolpin 2002).
5 Triclosan's toxic breakdown product methyl triclosan has been detected in fish downstream of wastewater treatment plants (Balmer 2004), and studies indicate that it persists in sediment for at least 40 years (Miller 2008). Though its widespread use and persistence in the environment indicate it might pollute tap water and food, testing is not required and no studies on this have been published.
6 EWG researchers compiled a comprehensive listing of 140 types of consumer products that can contain triclosan, from technical information and pesticide labels published by EPA [see the data], and from EWG's in-house database of ingredient listings on 30,000 personal care products (EWG 2008).
7 In a survey of personal care product ingredient labels, EWG found triclosan in 112 of 259 liquid hand soaps (43%) and 47 of 609 toothpastes (7%). The data are drawn from EWG's personal care product ingredient database (EWG 2008).
Triclosan No Better Than Plain Soap
The American Medical Association, Food and Drug Administration, and at least 40 researchers from 13 universities and public institutions worldwide have concluded that antimicrobial soap does not work any better than plain soap and water at preventing the spread of infections or reducing bacteria on the skin, according to our survey of the scientific literature and published agency positions. Findings from each of these sources are provided below.
American Medical Association, 2002
"Despite the recent substantial increase in the use of antimicrobial ingredients in consumer products, the effects of this practice have not been studied extensively. No data support the efficacy or necessity of antimicrobial agents in such products, and a growing number of studies suggest increasing acquired bacterial resistance to them. Studies also suggest that acquired resistance to the antimicrobial agents used in consumer products may predispose bacteria to resistance against therapeutic antibiotics, but further research is needed. Considering available data and the critical nature of the antibiotic-resistance problem, it is prudent to avoid the use of antimicrobial agents in consumer products."
Tan L, Nielsen NH, Young DC, Trizna Z. 2002. Council on Scientific Affairs, American Medical Association. Use of antimicrobial agents in consumer products. Arch Dermatol. 138(8):1082-6.
Food and Drug Administration Nonprescription Drugs Advisory Committee, 2005
"The data we saw said handwashing was pretty effective, plain handwashing, and there was no data that I saw that was very convincing that antiseptic handwashing was substantially more effective."
Alastair Wood, M.D. (Committee Chair), FDA Non-Prescription Drugs Advisory Committee. October 20, 2005 meeting transcript p. 354-355. Available from
http://www.fda.gov/ohrms/dockets/ac/cder05.html#Nonprescription
Drugs.
University of Minnesota, 2008
“The two antimicrobial soaps tested showed minimal virus reduction ... which is similar to that obtained by washing hands without any soap ... These results indicate that triclosan-containing antimicrobial soaps … may be inadequate for preventing norovirus transmission.”
Lages SL, Ramakrishnan MA, Goyal SM. 2008. In-vivo efficacy of hand sanitisers against feline calicivirus: a surrogate for norovirus. J Hosp Infect. 68(2):159-63.
University of Michigan, Columbia University, and Tufts University School of Medicine, 2007
“Soaps containing triclosan within the range of concentrations commonly used in the community setting (0.1%-0.45% wt/vol) were no more effective than plain soap at preventing infectious illness symptoms and reducing bacterial levels on the hands.”
Aiello AE, Larson EL, Levy SB. 2007. Consumer antibacterial soaps: effective or just risky? Clin Infect Dis. 45 Suppl 2:S137-47.
Swiss Tropical Institute, Basel, Switzerland, 2007
“The benefit achieved by the addition of Triclosan was not statistically significant.”
“The results strongly argue for regular soap use against common dermatomycoses as a low-cost and effective treatment.”
Dinkela A, Ferié J, Mbata M, Schmid-Grendelmeier M, Hatz C. 2007. Efficacy of triclosan soap against superficial dermatomycoses: a double-blind clinical trial in 224 primary school-children in Kilombero District, Morogoro Region, Tanzania. Int J Dermatol. 46 Suppl 2:23-8.
Tokyo Metropolitan Institute of Public Health, 2006
“By washing one's hands, about 99% of the viruses can be removed, compared with simply rinsing one's hands in running water. Washing one's hands with alcohol, chlorhexidine, quaternary ammonium, or 3 other kinds of hand soaps (containing povidone-iodine, triclosan, and isopropylmethyl phenol, respectively), was also effective for removing viruses. These results suggest that washing one's hands may be an effective method of preventing viral gastroenteritis.”
Mori K, Hayashi Y, Noguchi Y, Kai A, Ohe K, Sakai S, Hara M, Morozumi S. 2006. [Effects of handwashing on Feline Calicivirus removal as Norovirus surrogate]. Kansenshogaku Zasshi. 80(5):496-500. [Article in Japanese]
University of Michigan, Tufts University School of Medicine, and Columbia University, 2005
“Currently, no evidence suggests that use of antibacterial soap containing 0.2% triclosan provides a benefit over plain soap in reducing bacterial counts and rate of infectious symptoms in generally healthy persons in the household setting.”
Aiello AE, Marshall B, Levy SB, Della-Latta P, Lin SX, Larson E. 2005. Antibacterial cleaning products and drug resistance. Emerg Infect Dis. 11(10): 1565-1570. Available from http://www.cdc.gov/ncidod/EID/vol11no10/04-1276.htm.
University of North Carolina Health Care System, University of Maryland, and Duke University, 2005
“Effective hand hygiene for high levels of viral contamination with a nonenveloped virus was best achieved by physical removal with a nonantimicrobial soap or tap water alone.”
Sickbert-Bennett EE, Weber DJ, Gergen-Teague MF, Sobsey MD, Samsa GP, Rutala WA. 2005. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control. 33(2):67-77.
Columbia University, University of Michigan, New York Presbyterian Hospital, and Tufts University School of Medicine, 2003
“Overall, there were no significant differences in pre-to-post handwashing counts at baseline (p = 0.41), but by the end of one year, post-wash counts were significantly lower than pre-wash (p = 0.000) for those using either antimicrobial or plain soap. There were no significant differences in mean log counts either before or after handwashing between those using the antimicrobial or plain soap at baseline or after a year of use (all p values > 0.28)... A single handwash had minimal effect on quantity of hand flora, but there were significant effects over time, regardless of whether antimicrobial or plain soap was used. In the absence of more definitive evidence, the risk-benefit ratio argues in favor of targeted rather than ubiquitous, general household use of antimicrobial soap.”
Larson E, Aiello A, Lee LV, Della-Latta P, Gomez-Duarte C, Lin S. 2003. Short- and long-term effects of handwashing with antimicrobial or plain soap in the community. J Community Health. 28(2):139-50.
Kansai Medical University, Osaka, Japan
“[Triclosan] was much less effective than hand soap against hand surface bacteria.”
Namura S, Nishijima S, McGinley KJ, Leyden JJ. 1993. A study of the efficacy of antimicrobial detergents for hand washing: using the full-hand touch plates method. J Dermatol. 20(2):88-93.
John Hopkins University School of Nursing and Columbia University, 1989
“There were no significant differences between products in mean log10 colony-forming units after the initial wash (p = 0.61), nor were there significant differences in products after 5 days among subjects washing six times per day.”
Larson E, Mayur K, Laughon BA. 1989. Influence of two handwashing frequencies on reduction in colonizing flora with three handwashing products used by health care personnel. Am J Infect Control. 17(2):83-8.
Researchers from Hospital Infection Research Laborabory, Birmingham England, 1988
“Alcoholic preparations, particularly n-propanol and isopropanol were the most effective showing LRs of 3.1-3.8. Chlorhexidine (LR 2.9) and povidone-iodine detergent preparations were significantly more effective than non-medicated soap (LR 2.1), but triclosan products were not.”
Ayliffe GA, Babb JR, Davies JG, Lilly HA. 1988. Hand disinfection: a comparison of various agents in laboratory and ward studies. J Hosp Infect. 11(3):226-43.
Triclosan in Your Home
Where can you find triclosan in your home?
Table 1. Triclosan can be found in more than 140 types of personal care and home products
EWG identified triclosan-containing products from 3 government databases, Skin Deep cosmetics database, and 61 industry documents [see representative examples]
| Where is triclosan found? | |
| Bedroom | Bed spreads6 Blankets1,5,6 Comforters6 Duvets6 Mattress and pillow coverings4,5 Mattress pads6 Mattresses2 Mattress and pillow ticking1,5,6 Pillow fillings and fiber fill1,6 Pillowcases6 Pillow shams6 Sheets5,6 Quilts and quilt fabric1,4 |
| Bathroom | Bathtubs and bathtub enclosures5 Bath accessories6 Bath rugs6 Caulking (tub and tile)5 No-slip (rubber) bath mats5 Shower curtains2 Shower stalls5 Tile1,4,5 Toilet covers and seats4,5,6 Toilet bowls and urinals2 Towels5,6 |
| Kitchen | Countertops5 Covers for tabletops and countertops1,4 Cutting boards5,6 Dishwashing liquid9 Food and condiment storage containers5,6 Food service wipes1,4 Napkins1,5,6 Sponges6 Storage containers for appliances2 Tablecloths6 Tabletops5 |
| Personal care products | Acne treatment3 Antiperspirant/deodorant3,9 Antiseptics and dressings for wound care2 Body spray3 Body wash/cleanser3,9 Combs5,6 Cosmetic containers1 Diaper cream3 Facial cleanser3,9 Facial moisturizer/treatment3 Facial tissues2 Hairbrushes - bristles and handles1,4,5,6 Hand lotion9 Incontinence care products1,4,5,6 Lipstick3,9 Liquid hand soap3 Razors2 Shave gel9 Toothbrushes - bristles and handles2 Toothpaste3,9 |
| Lawn and garden | Garden hose10 Mulch2 |
| Clothing and other apparel | Aprons (food service, home)1,4,5,6 Athletic shirts/athletic wear1,4,5,6 Baby bibs1,4,5 Boots7 Caps6 Clothing inner linings6 Clothing trim5 Coats (coats' shells and fill)6 Dresses1,4,5,6 Footwear5 General wear clothing5 Gloves (non-medical)1,4,5,6 Golf shirts6 Gowns (medical and consumer)1,4,5 Hosiery1,4,5,6 Intimate apparel1,4,5,6 Non-woven products6 Rain wear10 Running gear1,4,5 Shoe inner soles1,4 Shoe lining1,4 Shoes1,4 Sleepwear6 Socks1,4,5 Sports apparel1,4,5,6 Sweatshirts/t-shirts4 Uniforms (service, medical, dental, sports)1,4,5,6 Wet suits1,4,5,6 |
| Sports, parks and recreation | Automotive interiors5 Boating/marine equipment5 Convertible tops6 Exercise and gym equipment5 Playground equipment1,4 Playing cards6 Portable toilets1,4 Swimming pool liner10 Tents2 Toys1,4,5 Whirlpools5 |
| Home furnishings and materials | Air filter materials2 Awnings (indoor and outdoor)2 Blanket brushes4 Cloths5 Curtains5,6 Draperies5,6 Furniture fabrics and coverings2 Furniture foam cushions1,4 Handles and knobs5 HVAC coils8 Tarpaulins6 Wall coverings6 Window curtains5 |
| Cleaning products | Brooms6 Brushes1,4 Buckets6 Carpet shampoos2 Cleaning fluids9 Fabric softeners2 Floor waxes/sealers/emulsions2 Garbage bags/garbage can liners2 Garbage cans/waste bins2 Laundry detergents2 Mops4,6 Stone surface cleaners 9 Vacuum bags5 Vacuum cleaner components5 |
| Floor coverings | Door and floor mats1,4 Rugs and carpets2 |
| Storage containers and linings | Blanket bags1 Clothing storage containers1,4 Garment bags1,4 Tray liners4 |
| Other home products | Electronics storage containers5 Floral containers1,4,5 Humidifier components5 Instrument cases5 Pet bedding4 Pet shampoos9 Pet training pads4 Phones5 |
| In your wallet | Credit cards5 Currency (paper money)5 Driver's licenses1 Personnel cards1 |
| Miscellaneous | Cubicle curtains1,4 Food distribution carts1,4 Food service trays1,4 Menu materials1,4,5 |
| Medical | Bed pans4,5 Medical devices2 Medical and dental trays1 Wheels for medical carts1,4 X-ray holders1,4 |
| Construction & Consumer Product Materials | Adhesives2 Caulk2 Cellulosic material2 Coatings2 Concrete2 Fabric2 Films2 Floor drains5 Flooring (concrete, plastic, floor covering)2 Grease traps Grout2 Hosing5 Ink2 Insulation2 Paints2,5 Paper and paperboard products1,4 Paper mulch1,4 Plastic2 Plumbing (outer metallic coating)1,4,5 Polyethylene2 Polypropylene2 Polyurethane2 Rope2 Rubber2 Sealants2 Slurries2 Styrene2 Synthetic polymer2 Textiles2 Vinyl2 |
| Industrial | Appliance housings5 Air filters5 Air filtration media5 Carts5 Closure cans5 Conveyor belts2 Dye bath vats2 Earplugs5 Fire hoses2 Ice making equipment (water pans, piping, tubing, guards)2 Respirator mask components5 Tabletops5 Trays5 Wipes5 Wraps (silage)5 |
1 Ciba 2006
2 EPA 2008a
3 EWG 2008
4 Huntsman International 2007
5 Microban Products Company 1997, 2000
6 Sanitized Inc 1998
7 Thomson Research Associates 2007
8 Aeris Technologies 2007
9 US Department of Health and Human Services 2008
10 Sanitized Inc 2003
Necessary Changes
The Environmental Protection Agency has approved triclosan for use in 20 pesticide formulations applied to 140 types of consumer products, without ever assessing the safety of this pesticide for babies and children. Triclosan persists in the environment, breaks down into substances highly toxic to wildlife, pollutes the human body, and poses health risks that are barely studied and poorly understood.
A 2005 study conducted by the Environmental Working Group (2005) found an average of 200 industrial chemicals, pesticides and pollutants in 10 newborn babies tested. Triclosan is just one of many common pollutants now found in the human body. We need a system of public health protections that protects children and others who are vulnerable from health impacts that can stem from the diverse, lifelong pattern of exposures to industrial chemicals that each person in this country endures. Babies should not be born pre-polluted with industrial chemicals. And they certainly shouldn't be born into the world polluted with a hand soap pesticide that doesn't work any better than plain soap and water.
EWG calls on EPA to conduct a thorough risk assessment of triclosan that would protect the health of people and the environment alike. We recommend:
- EPA should immediately request all missing studies from the pesticide manufacters, to procure data that will allow the Agency to rigorously assess the safety of triclosan for the fetus, infant, child, and other vulnerable populations. The Agency should fully evaluate the safety of the highly toxic breakdown products of triclosan, especially for children and the environment.
- FDA should ban the use of triclosan in personal care products, including liquid hand soap, body wash and other cleansers, which have not been shown to provide benefits over plain soap and water in independent studies [list of publications].
- Consumers should avoid the use of triclosan-laden products whenever possible. Wash hands, children's toys and other bacteria-prone surfaces often, with plain soap and water, to control the spread of infection. EWG's Guide to Triclosan can help.
- Manufacturers should curtail their use of this toxic, persistent chemical in consumer products, voluntarily in advance of mandatory restrictions.
Ultimately, the government should establish a system of public health protections that requires chemical companies to prove their products are safe before they are sold, for children and other vulnerable groups. Until we have such a system, chemicals like triclosan will continue to slip through the cracks to pollute the environment and people across the country, posing potential health risks that are poorly studied and little understood.
EPA Study Gaps Leave Children at Risk
EPA recently published a new, draft risk assessment for the antibacterial pesticide triclosan, as required by the pesticide re-registration provisions in federal law (EPA 2008a). In this draft assessment, EPA notes dozens of data gaps and uncertainties in triclosan exposure and safety studies, yet deems the available data "adequate for regulatory purposes," and bases all assessments of health and environmental risks on this deficient collection of studies. They've concluded that triclosan is safe for all consumer product uses. We question the validity of this conclusion.
Triclosan was long thought to pose little risk to people, since it works by suppressing production of a bacterial growth enzyme that humans don't have. But studies conducted over the past decade cast substantial doubt on that assumption. Widespread use of triclosan in over 140 everyday products, as well as detection of triclosan in the bodies of 75% of Americans (Calafat 2008), and in 58% of American streams and rivers (Kolpin 2002), makes a robust risk assessment of this pesticide essential to protecting human health and the environment.
EPA's cursory evaluation of exposures and health concerns associated with this antimicrobial agent could leave the public at risk. EWG has identified major gaps throughout the draft documents published in May (EPA 2008a), as detailed in a letter delivered to EPA on July 7, 2008. Specifically, EPA’s risk assessment:
- Lacks full assessment of risk for young children, despite the unique exposures that infants and young children receive through contaminated breast milk, house dust, contaminated food, toys, bibs and children's clothing, and children's body care products and toothpaste. Triclosan poses particular risks to infants and young children, as a laboratory study of developmental toxicity linked in utero exposures to the chemical to reduced fetal weight and irregular skull formation (MRID 43817501: citation missing from EPA's Preliminary Risk Assessment for Pesticide Uses of Triclosan (EPA 2008b)). Other studies link the pesticide to disruption of both the thyroid system and levels of calcium ion in the body (Veldhoen 2006; Ahn 2008), which could impact growth and development especially with respect to the brain and nervous system.
The two most glaring data gaps in EPA's evaluation of children's exposures to triclosan: use of an exposure dataset that does not include any information for Americans under 6 years of age (Calafat 2008), and complete disregard of the exposures infants receive through breast milk. A recent study indicates 97% of breast milk samples collected in the U.S. were contaminated with triclosan (Dayan 2007). Basic intake estimates for breastfed infants indicate that daily intake would be several times greater than exposures for older children (see EWG letter to EPA). - Does not provide an adequate margin of safety for children. The Agency's failure to provide an additional safety margin of 10 to account for potential neurodevelopmental effects and other impacts to which children may be especially vulnerable violates the intent of the nation’s pesticide law and leaves children at potential risk.
Clearly missing from EPA's inadequate triclosan health and safety data is a study on the developmental neurotoxicity of triclosan. With no research probing this important potential health concern, and with evidence from other studies indicating triclosan could impact functions essential to brain and nervous system development, EPA's supposition that children do not require extra protections is entirely unwarranted. - Neglects full assessment of inhalation risks and other cumulative exposures to triclosan. EPA must assess risk from the full range of Americans' inhalation exposures, both residential and occupational, since studies show that triclosan is most toxic when it is inhaled, with harmful effects at every dose tested.
Notably, EPA admits that inhalation studies have failed to find a safe dose; they show harmful effects from triclosan at all levels examined. Yet instead of requiring triclosan manufacturers to provide the studies needed to determine the safe dose, as EPA is empowered to do under pesticide law, the Agency has chosen to assess health risks using the flawed data, justifying their finding of safety by incorporating an additional fudge factor in their calculations to account for the data gap.
In the home, average Americans can inhale triclosan when they sleep on a triclosan-infused pillowcase, pillow or mattress; when they breathe in contaminated house dust; when they spray triclosan-treated paint; or when they use spray and powdered personal care products. We identified 114 triclosan-containing, inhalable products like spray deodorants and body powders in EWG's Skin Deep cosmetics safety database (EWG 2008). - Ignores numerous triclosan risks to human health. The Agency based its health risk calculations on an animal study showing no adverse health effects at a level that is 4.6 times higher than that reported for a study documenting triclosan's ability to cause liver toxicity in mice (Trutter 1993; EPA 2008a). The Agency's cancer risk assessment relied on second-hand information rather than actual study data (See 1996), and involves a controversial assumption that some laboratory animal cancers are not relevant to human health (EPA 2008c). EPA further neglected any assessment of triclosan's potential to disrupt the endocrine system, despite a growing body of research indicating that the pesticide may affect thyroid and reproductive hormone systems (Foran 2000; Ishibashi 2004; Matsumura 2005; Veldhoen 2006; Ahn 2008; Gee 2008).
Undoubtedly the most egregious data gap in EPA's assessment of human health data is its lack of access to critical cancer data. Liver adenomas and carcinomas were reported in a Colgate study of triclosan's effects on mice (See 1996), which the company refused to release to EPA. Lacking legal authority to demand the results, EPA was limited to reviewing a simple summary of information provided by FDA. As EPA conducted their new risk assessment, in the absence of Colgate’s cancer study data, the Agency chose to assume that the liver tumors observed were caused by a mechanism that doesn’t apply to humans, though there is no scientific consensus on this subject (Klaunig 2003; Peters 2005; Keshava 2006; NAS 2008). Rather than acknowledging the limitations of the health and safety information at its disposal, EPA repeatedly chose to make assumptions that minimize risks in its draft assessment of triclosan. - Lacks full assessment of risks to the environment. Triclosan is known to have significant acute effects on algae and other aquatic organisms at low levels, and is widely detected in streams across the U.S. In fact, triclosan is so toxic to aquatic life that EPA requires all triclosan raw materials to be labeled with this warning: "This pesticide is toxic to fish. Do not discharge effluent containing this product into lakes, streams, ponds, estuaries, oceans... Do not discharge effluent containing this product into sewer systems without previously notifying the local sewage treatment plant authority." Despite these facts, EPA's environmental risk assessment relies on a sparse group of ecotoxicological studies, and concludes that current uses pose no threat to the environment.
EPA's collection of environmental data on triclosan is riddled with holes (EPA 2008a). First, EPA could find no studies that assessed chronic ecotoxicological effects of the pesticide. Further, EPA's evaluation identifies data gaps including the lack of acceptable studies of triclosan's acute effects on freshwater invertebrates, marine organisms, and selected plants. The Agency finds the potential for triclosan use to overlap with endangered species habitat, noting that a more refined analysis is needed, but not included, in its draft assessment.
Triclosan persists in the environment for decades; a recent investigation revealed the presence of triclosan initially discharged 40 years ago in estuarine sediment in Chesapeake Bay (Miller 2008). Yet EPA disregards the effects of build-up of this pesticide in soils and sediments across the country. The Agency also ignores the risks posed by triclosan that contaminates biosolids applied to agricultural lands. EPA further neglects to assess the impact of exposure of low levels of antimicrobial agents like triclosan on the biological wastewater treatment systems essential to preserving the water quality of rivers and lakes throughout the country, as well as clean water supplies for downstream communities. Wastewater treatment plant operators fear that triclosan and other common antimicrobials in wastewater will begin to kill off the bacteria that break down human waste at the plant and are essential to cleaning it so that it can be discharged back to the environment (Tri-TAC 2008). - Ignores risks associated with toxic transformation products of triclosan. Triclosan breaks down or is transformed into very toxic chemicals, including a form of dioxin when sunlight hits it (Lores 2005); a chemical called methyl triclosan that is acutely toxic to aquatic life and is now found in rivers and streams the world over (Adolfsson-Erici 2002; Lindstrom 2002; Balmer 2004; Farré 2008); and the cancer-causing chemical chloroform when triclosan mixes with tap water that has been disinfected with chlorine (Fiss 2007).
While EPA mentions methyl triclosan in its risk assessment, it makes no attempt to evaluate exposure or risk associated with this chemical (EPA 2008a). Other transformation products are entirely neglected. This appalling lack of attention to commonly observed toxic by-products of triclosan violates U.S. pesticide law. - Neglects risks relating to antimicrobial resistance. In the draft assessment, EPA has not assessed potential health risks that could result through development of antimicrobial resistance to triclosan. Laboratory evidence and common sense indicate that microbes can develop resistance to the antibiotic effects of triclosan, rendering the pesticide ineffective.
EWG scientists assessed the growing body of evidence that indicates household use of triclosan could lead to the development of "super germs" and found clear cause for concern. Yet EPA made no attempt to evaluate this critical risk linked to unnecessary use of a pesticide that government agencies and leading scientists find does not provide the germ-fighting benefits that manufacturers of consumer and personal care products claim.
A risk assessment plagued by so many egregious flaws cannot be used as a basis for sound health protections. It is critical that EPA amend its assessment to fully evaluate a pesticide found in the bodies of three-quarters of Americans (Calafat 2008).
EPA's risk assessment of triclosan, necessary to reapprove its use as a pesticide, was limited to products under their jurisdiction. As such it does not address the safety of numerous FDA-approved body care products, including liquid hand soap, toothpaste and hand sanitizers. Though an advisory committee to FDA has found household use of "antibacterial" hand soap provides no health benefits over soap and water (FDA 2005), FDA continues to allow extensive application of the ingredient in a wide range of products. FDA is not obligated to consider the findings of EPA's investigation in any way, a failing plainly linked to fragmentation within the outdated system of chemical health protections in the U.S. Triclosan provides a clear case study supporting the need for reform of federal laws on toxic chemicals.
To take action now, EWG advises concerned consumers to avoid using "antibacterial" products. Soap and water provide the safest defense against germs in everyday life. Learn more from our Guide to Triclosan. You can check Skin Deep, EWG's cosmetics safety database, for body care products free of triclosan. Also avoid triclosan's chemical cousin, triclocarban. Other types of consumer products do not usually have ingredient lists – in this case, it's safest to avoid any product that makes "antibacterial" marketing claims.
Triclosan Toxicity
Taken together, many studies now demonstrate that triclosan is certainly not the safe and healthy bacteria-fighting hand soap ingredient we once might have assumed.
Triclosan: Toxic to people and the environment
CDC research on a broad cross-section of the population detected triclosan in the urine of 75% of 2,517 Americans (Calafat 2007). Higher levels of triclosan were typically found in higher income participants. An earlier study spearheaded by the Mount Sinai School of Medicine found triclosan in the urine of 61% of 90 girls age 6 to 8 (Wolff 2007).
Triclosan tends to bioaccumulate (Samsøe-Petersen 2003), or become more concentrated in the fatty tissues of humans and other animals. As a result, this chemical has been detected in human breast milk, and in blood samples as well (Adolfsson-Erici 2002; TNO 2005; Allmyr 2006a,b; Dayan 2007). Higher levels of triclosan in blood and breast milk are linked to use of body care products containing triclosan (Allmyr 2006b).
Lab studies link triclosan to cancer, developmental defects, and liver and inhalation toxicity. A secret study by Colgate scientists revealed exposure to low levels of triclosan caused liver tumors in mice (See 1996). Colgate refuses to release this study to EPA for evaluation, though it provided it to FDA in order to ensure it could add triclosan to toothpaste and other oral care products. Based on the study summary alone, and using a controversial assumption about the way this type of liver tumor forms in mice, EPA classified triclosan as “not likely to be carcinogenic to humans” (EPA 2008). This decision flows in part from EPA’s lack of regulatory authority to demand release of Colgate’s findings, a clear indication of the need for reform of the U.S. system of chemical health protections.
EPA does have access to several other lab animal studies linking triclosan to a variety of health effects. A study linking low level maternal triclosan exposure in mice to health effects in offspring, including irregular skull development and decreased fetal weight, provides evidence that triclosan may be a developmental toxicant (MRID 43817501: citation missing from EPA 2008b). Another mouse study, involving exposures to low levels of triclosan for 28 days, documented its toxic effects on the liver (Trutter 1993). While EPA summarizes the results of these studies in its documents, the Agency seems to ignore them when assessing the risks associated with this pesticide. In contrast, EPA acknowledges the substantial inhalation risk associated with triclosan, revealed by a 21-day rat study that found signs of toxicity at all levels of exposure (MRID 0087996: citation missing from EPA 2008b).
Triclosan may affect the thyroid and other hormone systems
A study of frogs shows that this pesticide perturbs a fundamental thyroid hormone signaling mechanism that is nearly identical to that of humans. Low levels of triclosan, in combination with thyroid hormones, triggered accelerated transformation of tadpoles into frogs (Veldhoen 2006). Triclosan, in concentrations under 1 part per billion commonly measured in U.S. streams, interfered with the timing of expression of thyroid-regulated genes that are crucial in a frog’s early development. Thyroid hormones are critical for normal growth and development of humans as well; the developing brain of a child is particularly vulnerable to damage caused by disruption of the thyroid system.
Triclosan may also disrupt other critical hormone systems. A recent lab study found the chemical to exert both estrogenic and androgenic effects on human breast cancer cells (Gee 2008). Studies of fish suggest that triclosan may have weak androgenic (Foran 2000) or anti-estrogenic effects (Matsumura 2005), while a metabolite of triclosan may have estrogenic effects (Ishibashi 2004).
Triclosan contaminates streams and is toxic to wildlife
Scientists recently found trace levels of triclosan in 58% of 85 streams located throughout the U.S. (Kolpin 2002), the likely result of its presence in discharges of treated wastewater. The pesticide has also been detected in several aquatic species (Remberger 2002; Adolfsson-Erici 2002). The amount of triclosan in wastewater is estimated to be as much as 3 to 5 milligrams per person per day from residences alone (McAvoy 2002); in addition, substantial discharges are expected from laundries, hair salons, medical facilities, and other sites. Optimized water treatment can remove up to 95% of triclosan (Samsøe-Petersen 2003); however, EWG research confirms that some triclosan persists despite treatment and enters receiving waters (EWG/EBMUD 2007). Triclosan is acutely toxic to several different types of aquatic life (e.g. Samsøe-Petersen 2003; Orvos 2002; Ishibashi 2004; Dussault 2008). Algae have proved to be the most sensitive organisms, but fish and invertebrates also experience adverse impacts following acute or chronic exposures to low levels of triclosan. An investigation of one algal species revealed genotoxic effects that warrant further study (Ciniglia 2005).
Triclosan forms other dangerous compounds
Studies indicate that in surface waters, triclosan can interact with sunlight and microbes to form methyl triclosan, a chemical that may bioacummulate in wildlife and humans (Adolfsson-Erici 2002; Lindstrom 2002). A recent European study found methyl triclosan in fish, especially concentrated in fatty tissue (Balmer 2004). Few studies have probed the toxicological effects of methyl triclosan, but a recent publication reveals that the transformation product triggers acute toxic effects in the marine bacterium Vibrio fischeri at levels significantly lower than does triclosan (Farré 2008).
Triclosan also can degrade into a form of dioxin, a class of chemicals linked to a broad range of toxicities including cancer (Lores 2005). The Canadian government limits the levels of dioxins allowed as impurities in personal care products that contain triclosan. New research shows that triclosan in tap water can react with residual chlorine from standard water disinfecting procedures to form a variety of chlorinated byproducts at low levels, including chloroform, a suspected human carcinogen (Fiss 2007).
Triclosan: One of many contaminants
Triclosan is just one of thousands of industrial chemicals in use in the U.S. today. As a pesticide, triclosan is subject to more stringent health and safety standards than many other industrial chemicals. Yet, as EPA’s flawed risk assessment makes clear, a few additional requirements do not necessarily result in chemical safety. Instead, what's needed is a new, comprehensive U.S. chemicals policy that truly protects children and other vulnerable populations from harm.
The Specter of "Super Germs"
Scientists and doctors have raised many and frequent concerns about the potential development of antibiotic-resistant bacteria as a consequence of the overuse of triclosan (Levy 2001; Tan 2002; Aiello 2005). In a recent publication, the American Medical Association (AMA) notes that "[s]tudies also suggest that acquired resistance to the antimicrobial agents used in consumer products may predispose bacteria to resistance against therapeutic antibiotics, but further research is needed. Considering available data and the critical nature of the antibiotic-resistance problem, it is prudent to avoid the use of antimicrobial agents in consumer products" (Tan 2002).
Researchers have not yet proven conclusively that triclosan-resistant bacteria are developing in homes and hospitals, but this is an all too common phenomenon for pesticides and antibiotics in general. Whenever bacteria are deluged with a pesticide or drug, mutant bacteria resistant to the pesticide have a selective growth advantage. Those resistant bacteria then proliferate and can become the dominant form. In recent years we have witnessed entire lines of drugs or pesticides lose effectiveness, leaving everybody more vulnerable to infection.
The phenomenon of reduced susceptibility is already apparent in several cases involving triclosan in laboratory studies (McMurry 1998; Chuanchuen 2001). These studies' identification of the triclosan-resistant bacterial enzyme suggests that resistance to triclosan may develop more readily than to other antibacterial agents (Heath 2000). In addition, exposing specific bacterial strains to triclosan appears to result in selection favoring bacteria that are resistant to multiple antibiotics (Chuanchuen 2001). These findings have led AMA to suggest that antibacterial products not be used in homes for fear that they would perpetuate the resistant strains of bacteria (Tan 2002).
Some scientists also suggest widespread household use of triclosan may result in increased risk of allergy in children, an unintended consequence potentially caused by alterations to a developing body's natural microbial community following chronic exposure to the pesticide, and resulting in immune system abnormalities (Levy 2001). While some advocate that triclosan plays a valuable role in the care of vulnerable patients in the hospital setting, even medical uses of triclosan may be called into question. A recent investigation of sutures coated with the pesticide, marketed as a means of preventing infection, found more complications developed for wounds closed with the triclosan-treated materials than for those closed with ordinary sutures (Deliaert 2008).
In summary, the best available science, joined with everything we have learned about pesticides, points to a single conclusion: Unless the use of triclosan is absolutely warranted, as it may be in specific clinical settings, our best bet is not to use it. Indiscriminate addition of triclosan to home-use products is unnecessary, does not appear to provide any additional protection from infection, and may breed triclosan-resistant "super germs." Let's act with common sense and save this potent chemical for times when we really need it.
EPA Approves 11 Companies' 20 Mixtures
EPA has approved 20 triclosan mixtures from 11 companies for use in products
As of July 2008, EPA’s pesticide registry lists 11 different manufacturers of 20 different triclosan formulations (NPIRS 2008). The pesticide is sold under a bewildering variety of trade names: Vinyzene DP 7000, Vinyzene SB-30, Sanitized Brand, Microbanish R, Vikol THP, Ultra-Fresh, Microban Additive “B”, Irgasan DP 300R, Irgaguard B 1000, VIV-20, AerisGuard, Ciba Tinosan AM110 Antimicrobial, Invasan DP 300R, and Invasan DP 300 TEX. In the late 1990s, the annual production volume of triclosan exceeded a million pounds and available data suggest it continues to grow (EPA 2002), a disconcerting trend considering the human health and environmental concerns associated with this pesticide. Shown below are the pesticide labels and technical data sheets for triclosan additives registered with the EPA. We collected these data and files from the National Pesticide Information Retrieval System (http://ppis.ceris.purdue.edu) and the EPA’s Pesticide Product Information System (http://www.epa.gov/opppmsd1/PPISdata/)
Sanitized Inc. (New Preston, CT)
Sanitized Brand T96-21 The oldest triclosan mixture still actively registered with the EPA, this additive, originally approved in 1969, is intended for use in apparel, active wear, hosiery, towels, and undergarments. EPA registration number 3090-165 Triclosan concentration 10%
Sanitized Brand PLA Approved in 1995, this additive is accepted for use in nearly 100 consumer products and residential/building materials. EPA registration number 3090-215 Triclosan concentration 99%
Sanitized Brand PL 91-36 Approved by the EPA in 2003, this additive is applied to articles and components made of plasticized PVC, such as floor and wall coverings, furniture fabrics, shower curtains, tents, garden hoses, and rain wear. EPA registration number 3090-219 Triclosan concentration 7.5%
S.C. Johnson & Son Inc. (Racine, WI)
Waste Minders with Stangard/4 EPA registration number 4822-429 Triclosan concentration 0.06% No records found in the EPA’s Pesticide Product Label System for this additive, first approved by the EPA in 1972.
Troy Chemical Corp. (Florham Park, NJ)
Microbanish R First registered with the EPA in 1997, this additive can be now found in products as diverse as shoe innersoles, carpets and rugs, window curtains and blanket bags. EPA registration number 5383-127 Triclosan concentration 99%
Vikon Chemical Co. (Lenoir, NC)
Vikol THP First approved by the EPA in 1972 and updated in 1997, this additive is applied to nylon, polyester, and acrylic textiles as well as blends of these fibers with cotton, rayon, or wool. EPA registration number 6390-25 Triclosan concentration 2%
Thomson Research Associates (Toronto, Canada)
Ultra-Fresh 300 DD Nonionic First approved by the EPA in 1972, this additive is intended for application to textiles, such as mattress covers, tickings and pads, pillow tickings, textile laundry additive and hosiery. EPA registration number 10466-24 Triclosan concentration 1.6%
Ultra-Fresh NM Approved in 1975, this additive is used for application to textiles and treatment of carpets, air filters, sponges, and cleaning cloths. EPA registration number 10466-27 Triclosan concentration 3%
Ultra-Fresh NM-100 Approved by the EPA in 1998, this additive is applied in polymers used for counter walls, sinks, and buried pipes. EPA registration number 10466-38 Triclosan concentration 99%
Ultra-Fresh FT-7 This additive for application to textiles and polymers was approved by the EPA in 2003. EPA registration number 10466-42 Triclosan concentration 1%
Microban Products Company (Huntersville, NC)
Microban Additive “B”: Microban 2000, Microban 1997 Originally approved by the EPA in 1983, this triclosan preparation is now listed as a potential additive in over 100 types of consumer products and home-use materials. EPA registration number 42182-1 Triclosan concentration 99%
Ciba Specialty Chemicals Corporation (High Point, NC)
Irgasan DP 300R First approved by the EPA in 1970, and still in active registration status, this additive is used for processing or manufacturing of treated articles. EPA registration number 70404-2 Triclosan concentration 99%
Irgaguard B 1000 Following EPA registration in 2000, this ingredient is now approved for use in nearly 100 polymer/plastic and textile-based products, including clothes, home furnishing and home-use consumer products. EPA registration number 70404-5 Triclosan concentration 99%
Vivimed Labs Limited (Hyderabad, India)
VIV-20 (triclosan) Approved in 2002, this bacteriostatic preparation is used for manufacturing, processing or repackaging of treated articles. EPA registration number 73951-1 Triclosan concentration 99%
Aeris Environmental Ltd. (Rosebury, New South Wales, Australia)
Aerisguard bioactive coil treatment Approved by the EPA in 2006, this additive is used for residential, commercial, and industrial application on HVAC coils. EPA registration number 82523-1 Triclosan concentration 0.69%
Huntsman International, LLC (The Woodlands, TX)
Ciba Tinosan AM 110 Antimicrobial EPA registration number 83884-7 Triclosan concentration 10% No records found in the EPA’s Pesticide Product Label System for this product, first approved in 1999.
Invasan DP 300R Following EPA registration in 2007, this ingredient is now approved for use in over 100 polymer/plastic and textile-based products, including clothes, home furnishing and home-use consumer products and products for pets. EPA registration number 83884-9 Triclosan concentration 99%
Invasan DP 300 TEX Following EPA registration in 2007, this ingredient is now approved for use in over 100 polymer/plastic and textile-based products, including clothes, home furnishing and home-use consumer products. EPA registration number 83884-10 Triclosan concentration 99%
Rohm and Haas Company (Philadelphia, PA)
Vinyzene DP 7000 Approved in 1994, EPA registration number 2829-139 Triclosan concentration 99%
Vinyzene SB-30 Approved in 1998, EPA registration number 2829-145 Triclosan concentration 10%





