 
Appendix: SLP test for assaying peptidoglycan
|
-Contents-
Establishment of Limulus test
Endotoxin-specific assays
Glucan-specific assays
Pretreatment methods of plasma
Plasma endotoxin levels in spsis and SIRS
Plasma endotoxin levels in other diseases than sepsis
Plasma glucan levels in depp mycoses and in some clinical situations
Why we should use endotoxin-specific limulus tests for assaying endotoxin in clincal samples
★Endotoxin activity assay (EAA)
REFERENCES
Establishment of limulus test
The name of "Limulus" is derived from a scientific name of the
American horseshoe crab; Limulus polyphemus. Four species of horseshoe crabs exist in limited regions of the world.
L. polyphemus is living in the East coast of America. Tachypleus tridentatus is living in coasts of Japan and China. T. gigas and Carcinoscorpius rotundicauda are found in the Western Pacific and Indian Ocean.
In 1956, Frederick B. Bang discovered that Gram-negative bacteria caused
the blood of a horseshoe crab, L. polyphemus, to clot (Ref. 1). He, with
Jack Levin, found that the lysate of amebocyte (circulating blood cells,
hemolymph) obtained by osmotic shock clotted on addition of purified endotoxin
(Ref. 2).
Quantitative Limulus tests
Prof. S. Iwanaga and his colleagues contributed to the development of quantitative
limulus test. They have found that the clotting enzyme and its substrate,
coagulogen, are involved in the gel fotmation. A quantitative limuls test
by adding a synthetic chromogenic substrate into the lysate was establised
by them (Ref. 3). The quantiative limulus test kit (Toxicolor) was commercialized
by Seikagaku Corporation Tokyo, Japan (Fig. 1).
In Toxicolor test, Boc-Leu-Gly-Arg-paranitroaniline (pNA) is used as a
synthetic chromogenic substrate for clotting enzyme. pNA released from
the substrate by the clotting enzyme is then diazocoupled to make a red
azodye (Ref. 3). Endotoxin content can be measured photometrically (545nm).
An alternative method is applied, such as a kinetic assay detecting the
increment of yellowish color of pNA.
Endotoxin-specific assays
As Prof. S. Iwanaga and his colleagues found the endotoxin and glucan involved pathway of the coagulation system (Ref. 4, 5, Fig. 1), Seikagaku Corporation (Tokyo, Japan) commercialized Endotoxin-specific test by removing factor G from the lysate of T. tridentatus (Fig. 1, Ref. 6). The lysate was fractionated by a chromatography and
reconstituted fractions other than factor G-containning fraction. This
endotoxin-specfic assay, Endospecy, is a chromogenic quantitative test,
like the conventional Toxicolor test.
Wako Pure Chemicals Industries Ltd. also established a quantitative assay,
turbidimetric kinetic assay; HS-test Wako. In this test, the increment
of turbidity due to gel-formation is calculated by a computerized equipment
(Toxinometer)(Fig. 2, Ref. 7).They established an endotoxin-specific limulus
test (ES-test Wako, Ref. 8), thereafter. This test adopted a previous report
showing that an excess amount of β-(1, 3)-D-glucan rather inhibits the
activation of factor G (Ref.9). The lisence of this endotoxin-specific test (Pyrostar ES-F) was approved by FDA in 2012!!
Fig. 1 Limulus cascade reaction
Fig. 2 Synthetic chromogenic assays: Three chromogenic assay; conventional test (Toxicolor), endotoxin-specific test (Endospecy), and glucan specific test (Gluspecy)(Seikagaku Corporation).
Fig. 3 Turbidimetric Kinetic Assay: Conventional (HS-test Wako) and endotoxin-specific
(ES-test Wako) assays.
For assaying β-(1, 3)-D-glucan, plasma is treated with a solution
containing polymyxin B and Triton X-100, and a conventional HS-test Wako
is used for the assay (β-(1, 3)-glucan-Test Wako).
Glucan-specific assays
Glucan-specific limulus tests was developed by deleting factor C fraction
from the lysate: Gluspecy, Seikagaku Corporation (Ref. 10).
Wako Pure Chemical Industries Ltd. developed an unique (β-(1, 3)-glucan
test by inhibiting the endotoxin activity in test specimen with polymyxin
B (Ref. 11) (Fig. 3).
Maruha Corporation, Tokyo, Japan also established a glucan-specific test
(B-G Star)(Ref.12).
Recently, FDA approved glucan-specific chromogenic limulus test (Glucatell,
ACC) for the diagnosis of fungemia.
We, with Immunology Institute, Tokyo, Japan, have developed monoclonal antibodies against activated factor C. The monoclonal antibodies were produced by a fusion with myeloma cells and mouse spleen cells immunized with LPS-factor C complex. Monoclonal antibodies which reacted with the complex but not with LPS or factor C were selected. When an appropriate amount of the monoclonal antibody was added to the lysate, the lysate responds only to glucan but not to LPS (Ref.13).
| Table Major Limulus tests available in Japan |
| Kit |
Company |
Specificity1) |
Method2) |
| Toxicolor |
Seikagaku3) |
E/G |
C |
| Endospecy |
Seikagaku |
E |
C |
| Gluspecy# |
Seikagaku |
G |
C |
| HS-test Wako |
Wako4) |
E/G |
T |
| ES-test Wako# |
Wako |
E |
T |
| β-D-glucan Test Wako# |
Wako4) |
G |
T |
| B-G Star# |
Maruha5) |
G |
C |
| QCL-1000, Kinetic QCL |
Ronza Japan6) |
E/G |
C |
| Pyrogent5000 |
Ronza Japan |
E/G |
T |
| PyroGene |
Ronza Japan |
E |
F |
| Endosafe-PTS |
Charles River7) |
E/G |
C |
1) E: Specific for endotoxin, G: Specific for glucan, E/G: Reacts with both endotoxin and glucan
2) C: synthetic chromogenic method, T: turbidimetric kinetic assay, F: fluorscence method
3) Seikagaku Corporation
4) Wako Pure Chemical Industries Ltd.
5) Maruha Corporation
6) Ronza Japan
7) Charles River
8) This test uses a conventional limulus lysate (HS-Test Wako). A test
sample is pretreated with polymyxin B to inhibite endotoxin activity.
#These tests are authorized by the Ministry of Health and Welfare of Japan
as being diagnostic tools for the health insurance adjustment.

Plasma pretreatment methods
Interfering factors, i.e., inhibitors and non-specific amidase activity
for limulus reaction, are present in human plasma, and it must be removed
before testing.
For this aim, a perchloric acid (PCA) method was devised for Toxicolor
and Endospecy (Ref. 14): PCA is added to plasma and the precipitate (proteins)
is discarded after centrifugation, and the supernatant is used for Endospecy
test.
However, we found that this method can not offer to measure protein-bound
endotoxin in plasma. Then we developed a devised perchloric acid method
( (New PCA method, Ref. 15, 16). This new method allows us to detect the
total content of endotoxin in the plasma. About 8 to 10 times the endotoxin
content and 1.4 times the glucan content can be measured by the new PCA
method which is more than by the PCA method.
A new method using an alkali reagent was thereafter developed for pretreatment
of plasma endotoxin (Ref. 17). This method was reported to be shown comparable
result with New PCA method. However, we pointed out the occurrence of false
positive reaction by the generation of turbidity at early time during incubation
in this method (Ref. 18). This method is no longer used subsequently for endotoxin assay.
Dilution and heating method has been widely used for limulus tests. Wako
Pure Chemical Industries Ltd. devlpoed an improved dilution and heating
method, for a turbidimetric kinetic assay. In this method 0.02% Triton
X-100 is used instead of water for the 10-times dilution of plasma, and
heating condition was set at 70 degree C for 10 min (Ref. 19).
Plasma endotoxin levels in sepsis and SIRS
We investigated the plasma endotoxin levels in many situations, using endotoxin-specific
limulus test (Endospecy) with the new PCA method. Cut-off value wa set
at 9.8 pg/ml by the measurement of endotoxin levels of volunteer's plasmas
(Ref. 20).
The plasama endotoxin value often exceeded the cut-off value in a sepsis, septic shock, and the typhoid fever (Ref. 20, 21).
As a conclusion, we have found that high plasma endotoxin levels are related
to local or systematic infection due to gram-negative microrganisms.
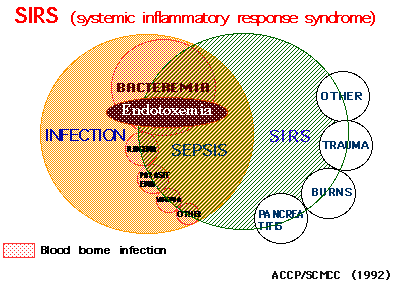
SIRS (systemic inflammatory response syndrome) is a proposed concept for
systemic inflammation in infectious and noninfectious conditions (Ref.
22). SIRS is thought to be due to the high cytokinemia induced by some
bacterial components. We concluded that endotoxemia was related to a part
of SIRS (Fig. 4).
Fig. 4 SIRS; Endtoxemia is associated mainly with bacteremia.
Plasma endotoxin levels in other diseases than sepsis
It has been reported that major stresses such as burns, massive trauma,or
hemorrhagic shock produce ischemia of the intestinal tract, which allows
endotoxin and gram-negative bacteria to enter the blood and gives rise
to endotoxemia.
There are reports as to endotoxemia in the early stages of burn injury, and as the burned area becomes larger the blood endotoxin level apparently increases.
However, we hardly found endotoxemia in severely burned patients at the
time of admission and in the first week after the burn injury whe using
the endotoxin-specific limulus test (Ref. 23). In 42 patients with burns
covering more than 20 percent of the body surface area, only one patient
had a plasma endotoxin level above 9.8 pg/ml at the time of admission,
and only 6 among 956 blood samples (0.70 %) showed endotoxemia at most
12 pg/ml.
Endotoxin levels increased more than 1 week after the burn injury when
infection had become established. It seems possible that infected burns,
and not the intestinal tract provided the source of endotoxemia.
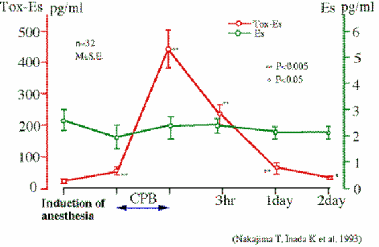
We have never found a patient with endotoxemia or a positive blood culture in the early stages of hemorrhagic shock (Ref. 24). During and after a cardiopulmonary bypass operation, endotoxemia was hardly found. On the otherhand, β-D-glucan or glucan-like activity was found in those plasmas (Ref.25, 26). Fig. 5 shows that β-D-glucan level (Toxicolor - Endospecy) rises during the operation. As this situation was not related to fungal infection, it is thought to be due to the generation of endogenous β-D-glucan-like activity.
Fig. 5 Endotoxemia does not occure in CBP
It has also been reported that cytokine levels are found to be elevated
after CBP and the rise was considered to relate to limulus test positivity.
We have more detailed data as to the rise of IL-6 and IL-8 levels (Ref.
27). It is likely that oxygen radicals (but not endotoxin) released in
an ischema reperfusion condition are related to the stimulation of cytokine
production.
In our other data, only the Toxicolor level rises, but not in the Endospecy test, after an esophageal varices operation (Ref. 28). In this situation, it seemed that fungal infection was not associated with the operation, therefore endogenous factor G-involved activity may be generated.
In normal delivery cases with no infectious signs, β-D-glucan was occasionally
detected in venous cord blood but not in maternal blood (Ref. 29).
In liver cirrhosis, sometimesthe Toxicolor level rises but the Endospecy
level slightly exeeded normal range (Ref. 30). We are postulating the existence
of an endogenous substance that activates factor G in liver diseases.
We found that a quantitative endotoxin assay in CSF is a helpful tool for
the diagnosis and prognosis of gram-negative meningitis (Ref. 31).
Very usefull diagnostic tool of plasma glucan level assay in deep mycoses
When we had no direct method for measuring blood β-D-glucan level, we were
regarded as the glucan level sustracting the Endospecy value from the Toxicoloe
value (Ref. 32, 33). The method was very usefull for the diagnosis of deep
mycoses (fungemia). β-D-glucan-specific limulus test was then developed,
diagnosis of fungemia has become easy. The incidence of fungemia is increasing
due to the increment of a compromised host as the result of higher quality
treatments. Super infection by fungi sometimes occurs after an intensive
therapy using antibiotics. Therefore, the importance of a glucan-specific
limulus test is increasing.
The plasma obtained from patients, who were dialyzed with a cellulosic
dialyzer or intravenously receiving anticancer polysaccharide, shows a
positive reaction in the conventional limulus test and glucan-specific
test but not in the endotoxin-specific test. This is thought to be due
to the β-D-glucan or substance(s), having a similar structure like glucan,
which is derived from the therapeutic procedure or substances.
It has been reported that other unknown endogenous substance(s) initiate the factor G pathway in some clinical or normal conditions (Ref. 26).
Why we should use endotoxin-specific limulus tests for assaying endotoxin in clincal samples?
Therefore, it should be noticed that the endotoxin value obtained by a
conventional limulus assay will lead to overestimate the role of endotoxin
in the mentioned disease. Thus, we should use endotoxin-specific limulus
test to evaluate the role of endotoxin in diseases. But unfortunately,
it is difficult to use that method, because the assay kit is not on the
market of the world, except in Japan. Recently, FDA approved a license
of endotoxin-specific limulus test (Pyrostar) of Japanese Company. In the
near future, the truth of the role of endotoxin in diseases will be opened
in the world.
★Endotoxin activity assay (EAA)
The endotoxin activity assay (EAA, Ref. 34) is a rapid chemiluminescent
immunodiagnostic test kit. EAA is a very interesting method for plasma
endotoxin detection, although this is not a limulus test. According to
my understanding, EAA is an indirect endotoxin assay, because EAA has many
steps, i.e., complex formation of LPS with anti-LPS IgM antibody, complement
activation, adherence of LPS/anti-LPS/C3b complex to CR1 and CR3 on the
surface of PMN, and activation of PMN to generate radical oxygens.
These steps will be influenced by many factors. For example, LPS binding substances or proteins will interfere the complex formation of LPS with anti-LPS IgM, and some protease inhibitor inhibits complement activation. The amounts of adhesion molecules may change in infectious diseases. PMN activity is also influenced by inflammatory situations.
Matsumoto, et al. (Ref. 35) recently found that EAA could not make dose-response
curve when 1-1000 pg/ml of LPS were spiked to normal human blood. They
also showed that IL-8 or TNF-alpha elevated EAA level when they were added
to normal human blood. Therefore, the commercial available EAA kit could
not measure endotoxin levels detected frequently in clinical situations.
Rather, EAA kit seemed to detect PMN with priming state.
References
1) Bang F B, A bacterial disease of Limulus polyphemus. Bull Johns Hopkins Hosp 98:325-51,1956
2) Levin J, Bang F B, The role of endotoxin in the extracellular coagulation
of Limulus blood. Bull Johns Hopkins Hosp 115: 265-74,1976
3) Iwanaga S, Morita T et al, Chromogenic substances for horseshoe crab
clotting enzyme. Its application for the assay of bacterial endotoxins.
Haemostasis 7:183-8,1978
3X) Obayashi T, Kawai T, Tamura H, Nakahara C (1982) New limulus amoebocyte
lysate test for endotoxemia. Lancet I:289
4) Morita T, Tanaka S et al, A new (1, 3)-β-D-glucan-mediated coagulation pathway found in Limulus amebocytes. FEBS lett 129:318-21,1981
5) Iwanaga S, Kawabata S, Evolution and physiology of defense molecules
with innate immunity in horseshoe crab.Frontiers in Bioscience 3:d973-84,1998
6) Obayashi T, Tamura S et al, A new chromogenic endotoxin-specific assay
using recombined limulus coagulation enzymes and its clinical applications.Clin
Chim Acta 149:55-65,1985
7) Oishi H, Takaoka A et al, Automated limulus amebocyte lysate (LAL) test
for endotoxin analysis using Toxinometer ET-201. J Parent Sci Technol 39:194-201,1985
8)Kambayash J, Yokota M, Sakon M, Shiba E, Kawasaki T, Mori T, Tsuchiya M, Oishi H, Matsuura S, A novel endotoxin-specific assay by turbidimetry with Limulus amoebocyte lysate containing β-glucan. J Biochem Biophysic Methods 22:93-100,1991
9) Kakinuma A, Asano T et al, Gelation of limulus amebocyte lysate by an
antitumor (1, 3)-β-D-glucan. Biochem Biophys Res Commun 101:434-9,1981
10)Obayashi T, Yoshida M, Tamura H, Aketagawa J, Tanaka S, Kawai T (1992) Determination of plasma (1→3)-β-D-glucan: a new diagnostic aid to deep mycosis. J Med Vet Mycol 30:275–280
11) Mori T, Ikemoto H et al, Clinical evaluation of plasma (1, 3)-β-D-glucan measurement by the kinetic turbidimetric Limulus test for the clinical diagnosis of mycotic infections. Eur J Clin Chem Clin Biochem 35:553-60,1997
12) Kitagawa T, Tsuboi I et al, Rapid method for preparing a β-glucan specific
sensitive fraction from Limulus(Tachipleus tridentatus) amebocyte lysate. J Chromato 567:267-73,1991
13) Yoshida M, Inada K et al, An assay method of (1, 3)-β-D-glucanto diagnose invasive mycosis. A utilization of monoclonal antibody to the activated factor C in blood coagulation system of horseshoe crab. In Fugal cells in biodefense mechanism (S. Suzuki & M. Suzuki eds), Saikon Publishing Co., Ltd. (Tokyo), pp265-71,1996
14) Obayashi T, Addition of perchloric acid to blood samples for colorimetric
limulus test using chromogenic substrate: Comparison with conventional
procedures and clinical applications. J Lab Clin Med 104:321-30,1984
15) Takahashi K, Study on quantitative measurement of endotoxin in human blood using chromogenic substrate. J Iwate Med Ass 40:67-81,1988
16) Inada K, Endo S et al, Establishment of a new perchloric acid treatment method to allow determination of the total endotoxin contentin human plasma by the limulus test and clinical application. Microbiol Immunol 35:303-14,1991
17) Tamura H, Arimoto Y et al, Automated kinetic assay for endotoxin and
(1, 3)-β-D-glucan in human blood. Clin Chim Acta 226:109-12,1994
18) Inada K, Endo S, Discrimination between specific and non-specific reactions of kinetic Limulus test for the measurement of endotoxin and β-glucans in blood. Igaku to Yakugaku 42:885–97,1999 (in Japanese)
19) Inada K, Yamashita H, Yoshida M, Harada K, Tsuchiya M, Matsuura S,
Evaluation of detergent-dilution and heating method for measurement of
endotoxin in plasma. Kiso-to-Rinsho 26:4545–50,1992 (in Japanese)
20) Endo S, Inada K et al, Two types of septic shock classified by the
plasma levels of endotoxin and cytokines. Circulatory Shock 38:264-74,1992
21) Suyasa I G N, Inada K et al: Endotoxemia in typhoid fever, KobeJ Med
Sci 41:175-86,1995
22) ACCP/SCMCC Consensus Conference Committee: Definitions for sepsis and
organ failure and guidlines for the use of innovative therapies in sepsis.
Chest 101,1644-55/Crit. Care Med 20, 864-74,1992
23) Endo S, Inada K et al, Are plasma endotoxin levels related to burn
size and prognosis. Burns 18:486-9,1992
24) Endo S, Inada K, Yamada Y et al, Plasma endotoxin and cytokine concentrations
in patients with hemorrhagic shock. Cri Care Med 22, 948-55,1994
25) Hosotubo K K, Nishijima M K et al, Presence of circulating β-glucan
during cardiopulmonary bypass, J Thorac Cardiovasc Surg 103:163-9,1992
26) Nakajima T, Mukaida M et al, Limulus test (factor G pathway) positive
substance during cardiopulmonary bypass, Nippon Geka Gakkai Zasshi 95:
893-8,1994 (in Japanese)
27) Kawamura T, Wakusawa R, et al, Elevation of cytokines during openheart
surgery with cardiopulmonary bypass: participation of interleukin 8 and
6 in reperfusion injury, Can J Anaesh 40:1016-21,1993
28) Kikuchi M, Watanabe M et al, Portaland peripheral endotoxins in patients
with esophageal varices undergoing surgery. Surg. Today 25:17-20,1995
29) Suda H, Usuki Y et al, Endotoxin and endotoxin-like substance in human
cord blood. Japan J Neonat 25:829-33, 1989
30) Yajima M, Fukuda I et al, Non-septic endotoxemia in cirrhotic patients. Gastroent Japon 24:262-9,1989
31) Ichinohe S, Inada K et al, Usefulness of endotoxin-specific limulustest
for the measurement of endotoxin in cerebrospinal fluid in diagnosis of
baecterial meningitis, J Japan Ass Infect Dis 69:1227-34,1995
32) Ikegami K, Ikemura K et al, Early diagnosis of invasive candidiasis
and rapid evaluation of antifungal therapy by combined use of conventional
chromogenic limulus test and a newly developed endotoxin specific assay.J
Trauma 28:1118-26,1988
33) Endo S, Inada K et al, Perchloric acid, Toxicolor, Endospecy, and miconazole
in the earl diagnosis and treatment of fungemia. Clinic Therapeut 12:323-6,1990
34) Romaschin AD, Harris DM, Ribeiro MB, Paice J, Foster DM, Walker PM,
Marshall JC, A rapid assay of endotoxin in whole blood using autologous
neutrophil dependent chemiluminescence. J Immunol Methods 212:169-85,1998
35) Matsumoto N, Takahashi G, Kojika M, Suzuki Y, Inoue Y, Inada K, Endo S, Interleukin-8 induces an elevation in the endotoxin activity assay (EAA) level: does the EAA truly measure the endotoxin level? J Infect Chemother 19:825-32,2013

Appendix: SLP test for assaying peptidoglycan
Last updated: 2010/01/25
Fig. Reaction cascade in SLP
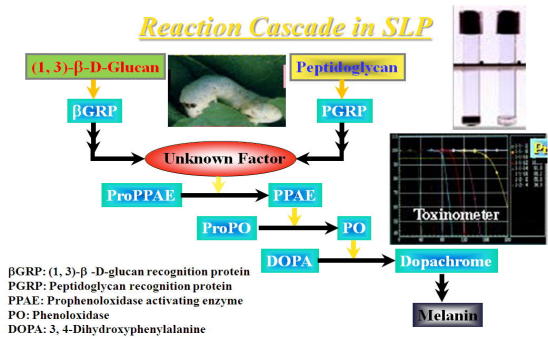
Peptidoglycan (PG) is a cell wall component of gram-positive and gram-negative
bacteria.The PG measuring method was developed by Tsuchiya, et al, by using
a silkworm larva plasma (SLP) (Ref. 1).
The SLP test is based on PG-induced melanin formation in SLP derived from
the hemolymph of silkworm, Bovyx mori (Fig). Interestingly, β-(1, 3)-D-glucan (GL) also forms melanin through
an alternative pathway.
The cascade reactions are preserved as a sclerosing, wound healing and
defensive reaction of the silkworm against foreign matter entering the
blood cavity (Ref. 2, 3).
A SLP reagent kit is available from Wako Pure Chemical Industries, Ltd., Osaka, Japan. SLP reagent consists with SLP and a substrate (3, 4-dihydroxy-phenylalanine; DOPA). One-hundred μl of the test sample is mixed with 100 μl of the SLP-substrate mixture, then incubated at 30oC for 120 min in the computerized instrument (Toxinometer, Wako Pure Chemicals
Industries, Ltd.).
During incubation, the melanin-formation (dark color generation) occurs
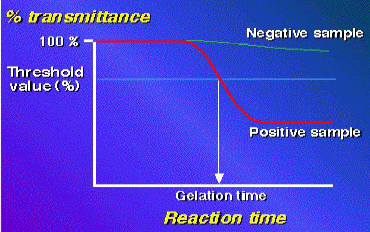
and the transmittance ratio decreases. The time (min; Ta) reaching to the
threshold value (95 %) of the transmittance ratio is measured. The content
of PG or GL is calculated with a log-log standard curve made by standard
PG or GL preparations.
Alternatively, dark color formation can visually be observed without the
computerized instrument.
A SLP test is thought to be useful for the diagnosis of bacterial infection. Previously we have confirmed that an endotoxin-specific limulus test is applicable for the diagnosis of meningitis due to gram-negative rods by measuring endotoxin in cerebrospinal fluid (CSF) (Ref. 4). Then, we applied SLP test to the diagnosis of bacterial meningitis due to gram-positive bacteria, gram-negative bacteria, or fungi (Ref. 5), and we found that CSF from patients with viral meningitis or noninfectious illnesses showed negative reaction.
Therefore, this test seems to be useful for diagnosis of bacterial and
fungal meningitis. When this test was used together with two types of limulus
tests, an endotoxin-specific test, and a conventional test, meningitis
was further characterized as gram-positive, gram-negative or fungal meningitis.
Kobayashi T. et al found that the SLP method provided a valuable tool for
the diagnosis of systemic infection using patients' blood (Ref. 6).
Plasma and patient CSF must be pretreated, because there are interfering
factors in the specimens to the SLP reaction. Dilution of a test specimen
with water is a most simple way, so far.
References
1) Tsuchiya M, Asahi N, Suzuoki F, AshidaM, Matsuura S, Detection of peptidoglycan and β-glucan with silk worm plasma. FEMS Immunol Med Microbiol 15:129-34,1996
2). Ashida M. Yamazaki HI, Biochemistry of the phenoloxidase system in
insects with special reference to its activation. In: Onishi E, Ishizaki
H (eds) Molting and metamorphosis. Japan. Sci. Soc. Press,Tokyo. pp239-65,
1990
3) Ashida M, Brey PT, Role of the integument in insect defense: Prophenoloxidase
cascade in the cuticular matrix. Proc Natl Acad Sci 92:10698-702,1995
4) Ichinohe S, Inada K, Nemoto T, Murata A, Ichinohe N, Fujiwara T, Yoshida
M, Usefulness of endotox in specific limulus tests for the measurement
of endotoxinin cerebrospinal fluid in diagnosis of bacterial meningitis.
Kansenshougaku Zasshi 69:1227-34, 1995 (inJapanese)
5) Inada K, Takahashi K, A silk worm larvae plasma test for detecting peptidoglycan
in cerebrospinal fluid is useful for the diagnosis of bacterial meningitis.
Microbio lImmunol 47:701-7,2002
6) Kobayashi T, Tani T, Yokota T, Kodama M, Detection of peptidoglycanin
human plasma using the silk worm larvae plasma test. FEMS Immunol Med Microbiol
28:49-53,2000


|
|
|
Copyright (C) Katsuya INADA 1997-2014, All rights reserved.
|
|