Christopher Mayne

Currently: Scientist II, Computational Chemistry @ Celgene
Home Department:
Beckman Institute for Advanced Science and Technology
Department of Chemistry
Office Address: 3011 Beckman Institute
Office Phone: (217) 300-6380
Email Address: mayne(at)ks(dot)uiuc(dot)edu
Education
- Post-Doctoral Research Associate, Tajkhorshid Laboratory, University of Illinois at Urbana-Champaign, 2011-2013
- Ph.D., Chemistry, University of Illinois at Urbana-Champaign, 2011
- B.S., Chemistry, University of Tennessee at Knoxville, 2004
Research Interests
Publications
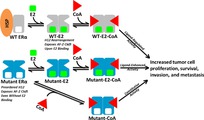
Fanning, S.W.; Jeselsohn, R.; Dharmarajan, V.; Mayne, C.G.; Karimi, M.; Buchwalter, G.; Houtman, R.; Toy, W.; Fowler, C.E.; Han, R.; Laine, M.; Carlson, K.E.; Martin, T.A.; Nowak, J.; Nwachukwu, J.C.; Hosfield, D.J.; Chandarlapaty, S.; Tajkhorshid, E.; Nettles, K.W.; Griffin, P.R.; Shen, Y.; Katzenellenbogen, J.A.; Brown, M.; Greene, G.L. (2018)
The SERM/SERD bazedoxifene disrupts ESR1 helix 12 to overcome acquired hormone resistance in breast cancer cells.
eLife, 7, e37161. (Accompanied by a "Related Insight" article)
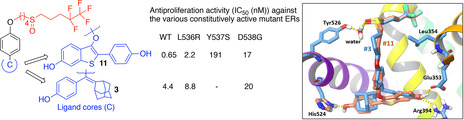
Sharma, A.; Toy, W.; Guillen, V.S.; Sharma, N.; Min, J.; Carlson, K.E.; Mayne, C.G.; Lin, S.; Sabio, M.; Greene, G.; Katzenellenbogen, B.S; Chandarlapaty, S.; Katzenellenbogen, J.A. (2018)
Antagonists for Constitutively Active Mutant Estrogen Receptors: Insights into the Roles of Antiestrogen-Core and Side-Chain.
ACS Chem. Biol. 13(12), 3374-3384.
Katzenellenbogen, J.A.; Mayne, C.G.; Katzenellenbogen, B.S.; Greene, G.L.; Chandarlapaty, S. (2018)
Structural underpinnings of oestrogen receptor mutations in endocrine therapy resistance.
Nat. Rev. Cancer 18, 377-388.
Speltz, T.E.*; Mayne, C.G.*; Fanning, S.E.; Siddiqui, Z.; Tajkhorshid, E.; Greene, G.L.; Moore, T.W. (2018)
A "Cross-Stitched" Peptide with Improved Helicity and Proteolytic Stability.
Org. Biomol. Chem., 16, 3702-3706.
Min, J.; Sanabria Guillen, V.; Sharma, A.; Zhao, Y.; Ziegler, Y.; Gong, P.; Mayne, C.G.; Srinivasan, S.; Kim, S-H.; Carlson, K.E.; Nettles, K.W.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. (2017)
Adamantyl Antiestrogens with Novel Side Chains Reveal a Spectrum of Activities in Suppressing Estrogen Receptor (ER)-Mediated Activities in Breast Cancer Cells.
J. Med. Chem., 60(14), 6321-6336.
Arcario, M.J.; Mayne, C.G.; Tajkhorshid,E. (2017)
A membrane-embedded pathway delivers general anesthetics to two interacting binding sites in the Gloeobacter violaceus Ion Channel.
J. Biol. Chem., 292(23), 9480-9492.
(Cover Article)
Mayne, C.G.; Arcario, M.J.; Mahinthichaichan, P.; Baylong, J.L.; Vermaas, J.V.; Navidpour, L.; Wen, P.-C.; Thangapandian, S.; Tajkhorshid,E. (2016)
The Cellular Membrane as a Mediator for Small Molecule Interaction with Membrane Proteins.
BBA-Biomembranes 1858(10), 2290-2304.
Vermaas, J.V.; Trebesch, N.; Mayne, C.G.; Thangapandian, S.; Baylon, J.; Jian, T.; Wang, Y.; Muller, M.P.; Shinn, E.; Zhao, Z.; Wen, P.-C.; Tajkhorshid,E. (2016)
Microscopic Characterization of Membrane Transport Function by In Silico Modeling and Simulation.
In Gregory A. Voth, editor: Methods in Enzymology, Vol 578: Computational Approaches for Studying Enzyme Mechanism Part B, MIE, UK: Academic Press, 2016, pp. 373-428.

Madak-Erdogan, Z.; Kim, S-H.; Gong, P.; Zhao, Y.C.; Zhang, H.; Chambliss, K.L.; Carlson, K.E.; Mayne, C.G.; Shaul, P.H.;
Korach, K.S.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. (2016)
Design of pathway preferential estrogens that provide beneficial metabolic and vascular effects without stimulating reproductive tissues.
Sci. Signal. 9(429), ra53.
(Cover Article,
Journal Podcast)
Teo, I.; Mayne, C.G.; Schulten, J; Lelièvre, T. (2016)
Adaptive multilevel splitting method for molecular dynamics calculation of benzamidine-trypsin dissociation time.
J. Chem. Theory Comput. 12(6), 2983-2989.
Perilla, J.R.; Hadden, J.A.; Goh, B.C.; Mayne, C.G.; Schulten, K. (2016)
All-Atom Molecular Dynamics of Virus Capsids as Drug Targets.
J. Phys. Chem. Lett. 7, 1836-1844.
(Cover Article)
Liu, Y.*; Singharoy, A.*; Mayne, C.G.*; Sengupta, A.; Raghavachari, K.; Schulten, K.; Flood, A.H. (2016)
Flexibility Coexists with Shape Persistence in Cyanostar Macrocycles.
J. Am. Chem. Soc. 138(14), 4843-4851.
Hosseinzadeh, P.; Mirts, E.; Pfister, T.; Gao, Y-G.; Mayne, C.G.; Robinson, H.; Tajkhorshid, E.; and Lu, Y. (2016)
Enhancing Mn(II)-binding and manganese peroxidase activity in a designed cytochrome c peroxidase through fine-tuning secondary sphere interactions.
Biochem. 55(10), 1494-1502.
Speltz, T.E; Fanning, S.W.*; Mayne, C.G.*; Fowler, C.; Tajkhorshid, E.; Greene, G.L.; Moore, T.W. (2016)
Stapled Peptides with γ-Methylated Hydrocarbon Chains for the Estrogen Receptor/Coactivator Interaction.
Angew. Chem. Int. Ed. 55, 4252.
("Hot Paper", Cover Article).
Fanning, S.W.*; Mayne, C.G.*; Dharmarajan, V.*; Carlson, K.E.; Martin, T.A.; Novick, S.J.; Toy, W.; Green, B.
Panchamukhi, S.; Katzenellenbogen, B.S.; Tajkhorshid, E.; Griffin, P.R.; Shen, Y.; Chandarlapaty, S.;
Katzenellenbogen, J.A.; Greene, G.L. (2016)
Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing
the activating function-2 binding conformation
eLife 5, e12792.
(Accompanied by a "Related Insight" Article).
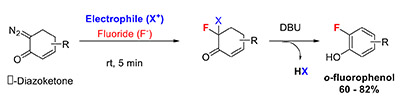
Yasui, N.; Mayne, C.G.; Katzenellenbogen, J.A. (2015)
Preparation of o-Fluorophenols from Nonaromatic Precursors: Mechanistic Considerations for Adaption to Fluorine-18 Radiolabeling
Org. Lett. 17(22), 5540-5543.
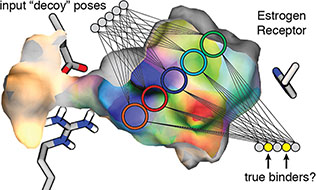
Durrant, J.D.; Carlson, K.E.; Martin, T.A.; Offutt, T.L.; Mayne, C.G.; Katzenellenbogen, J.A.; Amaro, R.E. (2015)
Neural-Network Scoring Functions Identify Structurally Novel Estrogen-Receptor Ligands
J. Chem. Inf. Model., 55(9), 1953-1961.
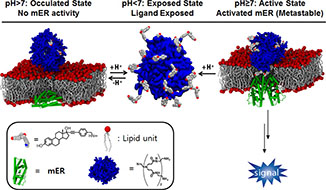
Kim, S-H.; Madak-Erdogan, Z.; Bae, S-C.; Carlson, K.E.; Mayne, C.G.; Granick, S.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. (2015)
Ligand Accessibility and Bioactivity of a Hormone-Dendrimer Conjugate Depend on pH and pH History
J. Am. Chem. Soc., 137(32), 10326-10335.
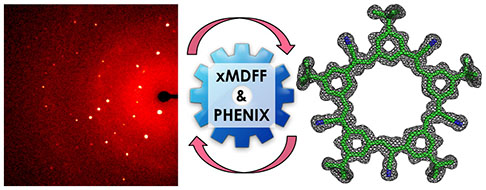
Singharoy, A.; Balasubramanian, V.; Liu, Y.; Mayne, C.G.; Lee, S.; Chen, C-H.; Zlotnick, A.; Schulten, K.; Flood, A.H. (2015)
Macromolecular Crystallography for Synthetic Abiological Molecules: Combinding xMDFF and PHENIX for Structure Determination of Cyanostar Macrocycles
J. Am. Chem. Soc., 137(27), 8810-8818.
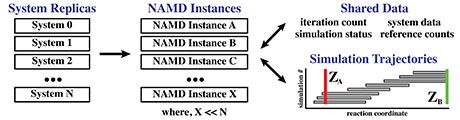
Aristoff, D.; Lelièvre, T.; Mayne, C.G.; Teo, I. (2015)
Adaptive Multilevel Splitting in Molecular Dynamics Simulations
ESAIM Proc., 48, 215-225.
Arcario, M.J.; Mayne, C.G.; Tajkhorshid, E. (2014)
Atomistic models of general anesthetics for use in in silico biological studies
J. Phys. Chem. B., 118, 12075-12086.
Mayne, C.G.; Saam, J.; Schulten, K.; Tajkhorshid, E.; Gumbart, J.C. (2013)
Rapid parameterization of small molecules using the Force Field Toolkit
J. Comput. Chem., 34, 2757-2770.
(Cover Article,
Top-Ten Most-Accessed Article 2014)
Moore, T.W.; Mayne, C.G.; Katzenellenbogen, J.A. (2010)
Not Picking Pockets: Nuclear Receptor Alternate-Site Modulators (NRASMs)
Mol. Endo., 24(4), 683-695.
(Cover Article)
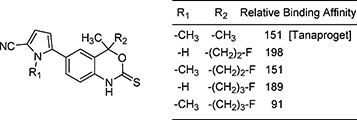
Zhou, H; Lee, J.H.; Mayne, C.G.; Carlson, K.E.; Katzenellenbogen, J.A. (2010)
Imagine progesterone receptor in breast tumors: Synthesis and receptor binding affinity of fluoroalkyl-substituted analogs of Tanaproget
J. Med. Chem., 53(8), 3349-3360.
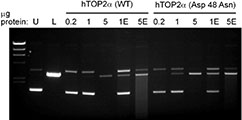
Walker, J.V.; Nitiss, K.C.; Jensen, L.H.; Mayne, C.G.; Hu, T.; Jensen, P.B.; Sehested, M.; Hsieh, T.; Nitiss, J.L. (2004)
A Mutation in Human Topoisomerase II α Whose Expression Is Lethal in DNA Repair-deficient Yeast Cells.
J. Biol. Chem., 275, 25947-25954.