Topic 6
Primary Vascular Tissues
xylem and phloem
Text
Objectives
- Identify and characterize the cellular elements of the xylem and phloem.
- Enumerate, evaluate, and describe the sources of the parallels between xylem and phloem on a developmental basis.
- Xylem and phloem are both complex tissues (more than one cell type is involved in each case).
- There are evident differences between xylem and phloem, but there are also important similarities.
- They have to be studied for their development in the primary plant body and in the secondary plant body.
The study of Secondary Vascular Tissues will lead us to a consideration of cambial activity (soon to come). In both primary and secondary vascular tissues it is important to understand development from meristematic cells to comprehend the sources of the similarities between xylem and phloem.
Objectives for this lab:
- To relate the range of wall patterns in tracheary elements to the maturation of tracheary elements relative to the axis that contains them.
- To apply practical criteria for visualizing the distinctions between primary and secondary vascular tissues as seen in sectional views.
- To utilize the experience obtained studying xylem to appreciate that similar relationships exist in the phloem between maturation of conducting elements and maturation of the plant axis.
The parallel treatment of xylem and phloem is purposeful and not accidental. Xylem is emphasized over phloem at this point to develop the concepts of proto- and meta- vascular elements, because xylem is easier for you to study. What you learn about stretching of cells in the xylem applies to the phloem but there is no diagnostic wall sculpturing to be followed in the phloem.
Additionally, the distortions of secondary walls in tracheary elements leave a clear record that some of the tracheary elements mature before elongation stops in the plant part that contains them; and the slowing and cessation of elongation correlates with the type of secondary thickening, suggesting at least a feedback mechanism or interplay between the growth of the axis and the details of maturation that occur in tracheary elements.
LABORATORY
This lab deals with primary vascular tissues and the transition to secondary vascular tissues. Some of your sections will show both xylem and phloem to good advantage simultaneously. If this happens, take advantage of the situation, but do not hesitate to concentrate on xylem first and phloem later.
Objectives:
- Identify a wide range of patterns in secondary wall thickenings in the tracheary elements of a plant axis.
- Recognize which of these patterns are subject to distortion by axis elongation.
- Determine to what extent a record of elongation is left behind when wall fragments are embedded in the maturing xylem.
- Identify sieve tube members in one or more plants.
Activity 1: A study of primary xylem.
- Start with a 10-minute study of Melilotus (sweet clover) stem. In the transverse section, identify the vascular bundles and study the portion of the xylem that is closest to the pith, looking for fragments of walls that are embedded among the cells of the region. One of the primary objectives will be to obtain a concept of what happened to produce these wall fragments. They are part of the protoxylem of the primary xylem. The late maturing primary xylem is called metaxylem.
On the radius from the center of the section through a vascular bundle, the protoxylem is toward the pith and protophloem is toward the epidermis. This pattern of xylem maturation is endarch. If the first-matured xylem were toward the middle or at the centrifugal locations, with respect to the xylem strand, the xylem would be characterized as mesarch, and exarch, respectively.
After orienting yourself with the transverse section, study the longitudinal section. Determine the types of wall thickenings in the primary xylem. See if you can detect wall fragments in the protoxylem region. Make sure you fully comprehend the orientation of the longitudinal section before making final determinations of what is present or absent in the primary xylem.
- The attenuation of the protoxylem is a matter of timing of maturation of the secondary walls in the protoxylem vs. the elongation of the plant axis after that maturation. A stretched helical thickening attenuates into a line, conspicuous because of its thickness and wavy shape. Produce a mechanical analogue of this attenuation from a piece of fresh Musa (banana) leaf, as follows:
If you have the chance of getting a Musa leaf, you can use your thumb and first finger of each hand, break the leaf gently across the veins, and slowly pull the fragments apart. The fine threads that emerge are helical secondary wall thickenings. The walls are deforming, not merely slipping out of the leaf. You can prove this because, if you are careful, you can stretch the threads to a dimension longer than the tissue they came from; and if you repeat the breaking of one of the fragments, then you can pull the threads as long the second time as the first.
Obtain a piece of leaf and draw out the threads over a drop of water, to a distance of about 1.5 cm. Set the fragments down on a slide on either side of the drop, so that the threads are in the water. Now add a coverslip and examine the mount. Look for coils, in the stretched and the relaxed position. Is there a resemblance between the stretched helix and what you saw in the longitudinal section of sweet clover? Are any of the helices double? Triple? For multiple helices, are the components tied together by small links? These are not easy to see! But if you find them, you have confirmed that the element in question was from metaxylem.
- Using Impatiens or Coleus make a first hand investigation of primary xylem in a fresh stem. You should strive to detect and identify various patterns of wall thickenings in each internode and to see how these patterns change (or are added to), as xylem matures across the bundles with aging of the internodes. A conceptual framework for what is happening in the internodes of real plants is given in figure below. You can use Toluidine Blue O (TBO) for staining your sections.
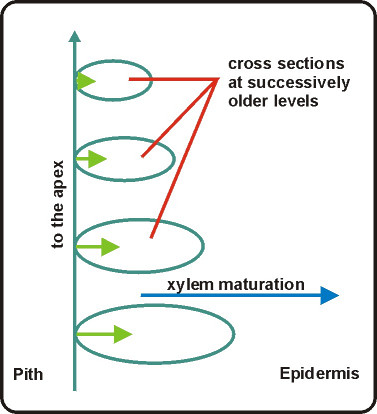
Gradients of xylem maturation: As a vascular bundle expands with age
the xylem matures further and further across the bundle. In the shoots of seed plants,
xylem maturation is from the pith side of the bundle toward the epidermis.This is not always a safe assumption; for example, flowering and aging affect shoot growth. A better way to approach the problem is to compare what appear to be the "same" internodes at different stages of growth, viz., to compare (for example) the fifth internode formed in seedlings of two different ages. In the older plant the internode will be older by virtue of the difference in planting date between old and young plant. Of course the whole series of internodes on the young plant can be examined in an older state by virtue of the analogous series of internodes in the older plant. Likewise, you could test for changes in the condition of the currently youngest internodes as a function of age of the plant, provided growth conditions have been uniform.
Proceed as follows with seedlings of different ages. As you make sections of older internodes they will be useful for Activity 2, so keep them!
- Arbitrarily designate the lowest internode on the younger plant as #1. Count upward to the smallest internode you can sample. Count upward on the older plant the same way to find this "same" internode. Now you have a series of paired internodes. The difference between members of a pair in the series gives the first possibility for comparison. Measure the lengths of "same" internodes (e.g., 1-5) on the two plants, and record the data in a table. If the plants are well suited to the comparison, a fully elongated internode on the younger plant should be about as long a fully elongated internode on the older plant, in a comparable position
- Start with the youngest internode that is convenient for handling (the younger, the better). With the shorter internodes, you may want to section the internode without detaching it from the axis. You don’t have to cut the internode off the plant to make these sections. With a transverse section for orientation, section the same piece of tissue longitudinally to show the primary xylem. Be sure to obtain sections in the proper radial orientation and passing through the bundles rather than between them. Keep the rest of the shoot moist while you work with each internode. Save the first slide for comparison with the others you make and diagnose the changes that may have taken place.
- Compare hand sections of the youngest internode with the "same" internode on the older plants.
- Work with successive pairs of internodes to extend the sample. This will develop a comparison up and down each plant as well as between plants. Then you can decide whether or not both comparisons lead to the same conclusion. To develop this point you can (optionally) sample right up to the top of the older plant, but save this for later if you feel that you still have time further on in the lab. Determine where each of these features occurs and how it fits into the aging (elongation) pattern:
- mature xylem elements present,
- stretched protoxylem elements present,
- annular, helical, reticulate patterns of secondary wall.
Strive for a concept of correlation between wall patterns and stretching of elements, and look for the details that contribute to the concept. The conviction that a certain element, say one with helical secondary walls, will or will not be stretched depends on the circumstances in which you find it. For a simple case, if pitted elements are present next to helical ones, then the helical ones would not be stretched any longer. On the other hand, if the last element to mature before you section the plant is helical, you would have no direct anatomical evidence on further stretching. Now you would have to predict whether the internode containing the element should undergo further elongation, at the level where you found the element. This is why measurements of internode length (and changes in length) would have to be a part of this kind of developmental study.
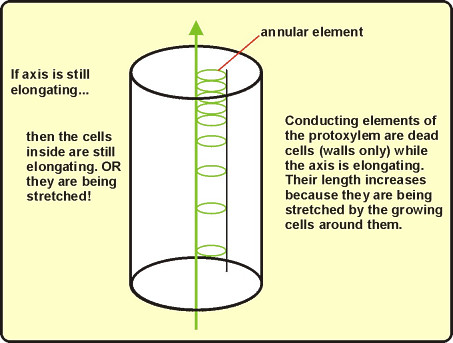
Elongation stretches protoxylem: Distortion of primary xylem walls leaves a record of previous
elongation. Destroyed elements are replaced by newly matured cells. As elongation tapers off,
metaxylem is formed, with the coordinated changes in wall patterns.NOTE:The next part of the study of primary xylem is based on prepared slides. You can come back to Activity 1C later if you wish to keep on working with your freehand sections. At any rate, do not discard your hand sections, yet, and do not let them dry out.
- Protoxylem lacunae and bundles without protoxylem. Use a Zea stem cross section. Destruction of the protoxylem elements in corn does not lead to the same morphological situation as seen after the destruction of the protoxylem
conducting elements in sweet clover. Instead a protoxylem lacuna is formed.
Examine the bundles near and away from the surface of the stem. Are they the same with respect to the development of a protoxylem lacuna? The largest bundles all have lacunae. The presence of the lacuna is positive evidence for protoxylem. Somewhat smaller bundles have seemingly intact protoxylem in the position occupied by the lacunae of larger bundles. In the smallest, most peripheral bundles, no conducting cells are found in the position where protoxylem elements are present in the larger bundles.
If bundles mature their first elements after elongation in the axis is over, protoxylem will be absent in those bundles. The smallest bundles are still procambium when elongation stops in any given part of the corn stem and do not elongate after maturation. In a system of bundles, as in a stem, protoxylem may be present in some bundles and absent in others at any level of sectioning.
For another situation where protoxylem may be lacking, look for bundles that offer little or no indication of stretching in clearings of Begonia leaf. These will be the tiniest veins and bundle endings. Compare these bundles with the major veins, which show ample record of stretched (proto-) xylem.
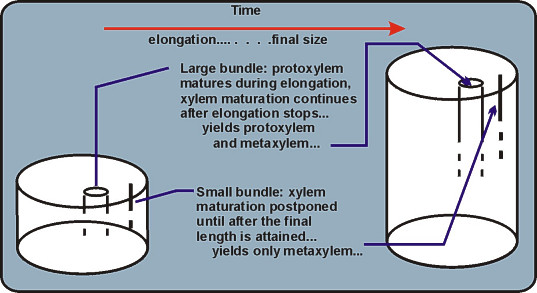
Protoxylem matures during elongation: Vascular bundles can lack protoxylem if their maturation is
prevented during elongation of the plant part they are within. The spatial pattern of arrangement of
bundles with and without protoxylem is a manifestation of coordination in development of plant parts and plant tissues.Realize from the beginning that these distinctions are a matter of definition for the sake of coherent description. Subjective judgments are involved in dealing with individual cases, as in the matter of recognizing procambium.
Activity 2: Identifying sieve plates as a diagnostic feature of phloem.
- Use unstained freehand sections of Impatiens, the sections you already have made, or check images. Your thinnest, most nearly radial longitudinal sections should be examined for phloem, which should be toward the exterior of the stem from the xylem. Look for a relatively colorless (white appearing) strip of tissue next to the xylem. The cells will be narrow compared to tracheary elements and relatively nondescript because they are so narrow. The primary phloem can be divided into proto- and metaphloem, and if your section shows secondary xylem, it probably also contains secondary phloem.
Sieve plates are specialized end walls. Try to find sieve plates in your material or images.
- Phloem in other species.
- Freehand sections can be made from Cucurbita (squash) to see extraordinarily wide sieve tubes with large pores in the sieve plates.
- Zea stems are useful for seeing the arrangement of sieve tubes and companion cells. Sieve plates can be identified in longitudinal sections.
- Sometimes the staining of callose or of sieve tube proteins (slime) can help identify phloem, but these substances are also studied to determine their effect on phloem transport. Wounding increases callose content in sieve elements and causes slime to surge up against the sieve plates. You will examine prepared slides stained for callose during your study of secondary phloem and you can also use one of your sections and stain it with Aniline blue.
- Wood Macerations: pick two already prepared slides and look for vessels and fibers. Observe the pitting and the perforation plates.
Questions
- Draw parallels and contrasts between primary xylem and primary phloem with regards to origin, development, maturation, cell types, etc.
- Outline the ontogeny of a vessel element and sieve tube member in the SAME plant. Contrast the end walls of these two kinds of conducting elements at maturation.
- What are the “peculiar” aspects of the protoplast of a sieve tube member? Do they relate to de function of the cell? Are similar peculiarities found on functional vessels elements? (Why or why not?)
- Describe a sieve plate (and any other special cytological features of sieve tube elements).
- What is the presumed role of the callose in cell walls and cell to cell interactions?
- What is a companion cell? What is its possible role on the phloem?
- Criticize and/or support the following statement: “Phloem is composed of several cell types, among them fibers which are associated to the periphery of the phloem. These fibers are known as phloem fibers.” (Hint: xylary fibers are only associated with the xylem, those fibers that are elsewhere are “extraxylary”.)
- Criticize and/or support the following statement: “Xylem and phloem are intimate related and one cannot survive without the other”. Support your idea explaining why?
- What is the full range of types of wall thickenings in primary xylem?
- Design an experiment to study changes in primary xylem- One paragraph will be sufficient.
- Elongation of the axis somehow influences wall thickenings in primary xylem. What is the outcome of early cessation of elongation with regard to the assortment of wall thickenings in primary xylem?
- Suggest a model that would describe how the rate of elongation could determine or affect the kinds of wall thickenings in primary xylem.
- Criticize and/or support the following statement: “It cannot be secondary xylem if there is not first primary xylem”.
- Criticize and/or support the following statement: “Protoxylem differentiates before they complete elongation. As a result, they are small-diameter cells with extensible wall thickening patterns (annular and helical) which can continue to elongate. Metaxylem differentiate after additional growth and elongation has occurred, therefore metaxylem tracheary elements are larger diameter cells with mostly reticulate, scalariform, and pitted wall patterns”.