|
|
|
|
 |
 |
| |
 |
|
|
Quarterly Reviews |
|
|
|
|
|
Keywords :
Submucosal lesion, endoscopic ultrasound, GIST, leiomyoma |
|
|
Vikram Bhatia1, Masahiro Tajika2, Archana Rastogi3
Departments of Medical Hepatology,1 and Pathology,3
Institute of Liver and Biliary Sciences,
New Delhi, India;
Department of Endoscopy, 2
Aichi Cancer Center Hospital,
Nagoya, Japan
Corresponding Author:
Dr. Vikram Bhatia
Email: vikrambhatiadr@hotmail.com
DOI:
http://dx.doi.org/
Abstract
Submucosal lesions (SML) include a diverse array of benign, potentially malignant, andmalignant lesions. The majority of SML’s are asymptomatic and found incidentally. Endosonography (EUS) is the key investigation for these lesions. Although, the morphologic appearance of a SML as seen on EUS is rarely diagnostic, the differential diagnosis can be narrowed down. Obtaining a tissue diagnosis is often necessary, and EUS-FNA and EUSguided trucut biopsy of a SML can be carried out. Information about the malignant potential, layer or origin, size, and extramural extension of an SML is also provided by EUS. EUS is strongly indicated before endoscopic or surgical resection of any SML. The most commonly encountered SML’s in the upper gastrointestinal tract are GIST’s, leiomyoma’s, neuroendocrine tumors, lipomas, granular cell tumors, varices, duplication cysts, heterotopic pancreas, Brunner’s gland hamartoma, lymphangiomas. A large number of rare lesions are also seen. This review describes the histological, clinical, endoscopic, and endosonographic appearance of the different SML.
|
48uep6bbphidvals|205 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext The term submucosal lesion (SML) describes any protuberance in the gastrointestinal tract covered with normal mucosa. However the surface of the lesion may sometimes be ulcerated or inflamed. SML’s includes a variety of neoplastic and non-neoplastic lesions originating from the deeper layers of the gastrointestinal tract wall, without direct involvement of the epithelial layer. Endoscopic ultrasound (EUS) can distinguish between extramural compression and a true SML, and provide information about the echoarchitecture and layer of origin of the lesion. This information when considered in the clinical context, may be sufficient for a definite diagnosis, or reduce the diagnostic possibilities to a few. EUS guided fine needle aspiration (FNA) or trucut biopsy can provide a tissue diagnosis of SML, when required.
Prevalence and autopsy studies
Majority of SML are asymptomatic and found incidentally. In a retrospective study of over 15,000 endoscopies done over 8 consecutive years (July 1976-June 1984), SML were found in 0.36% patients (approximately 1 in every 300 endoscopies)[1]. In a histopathological study of 342 cases (276 autopsy and 66 surgical), leiomyoma (s) of the esophagus were found in 27 (7.9%) cases. Most of the leiomyomas originated from the inner circular muscle layer, and all were small in size[2]. In a recent study of 150 patients who underwent esophagogastric resection for esophageal or esophago-gastric carcinoma, incidental gastrointestinal stromal tumors (GIST’s) and leiomyoma’s were found in 10% and 47% of patients, respectively. The size of GIST’s ranged from 0.2 mm to 3.0 mm, and the size of leiomyomas ranged from 0.2 mm to 12 mm. All these tumors were confirmed by immunophenotyping[3]. There is no prevalence or autopsy data for the other less common SML’s.
Extraluminal compression
Extraluminal compression is established on EUS when all 5-layers of the gastrointestinal tract are found between the gut lumen and the lesion. EUS is highly accurate for the differentiation of extraluminal compression from a submucosal tumor, and in the identification of the compressing organ[4]. In a prospective study of 150 patients with presumed SMT on endoscopy, 32% patients were found to have extraluminal compression by EUS. In most cases the compression was normal adjacent organs or structures. For differentiating between a SML and an extraluminal compression, the sensitivity and specificity of endoscopy were 87% and 29%, whereas those of EUS were 92% and 100%, respectively[5]. In 11 endosonographic series, including a total of 1181 predominantly gastric lesions, extraluminal compression was found in 29% of all patients with suspected SML[6].
The esophagus can have impression from the aortic arch, dilated left atrium, anomalous branches of the aortic arch, enlarged mediastinal lymph nodes, and mediastinal tumors (Figures 1A,1B,1C). A normal spleen usually makes an impression in the gastric fundus and upper body, and the gallbladder can impress upon the gastric antrum. Transient gastric impression can be caused by adjacent bowel loops. Other causes of gastric impression include splenic vessels, pancreas, left liver lobe, as well as extra-gastric tumors (Figure 2)[7].

SML location
The most common category of lesions presenting as SML are smooth muscle tumors (leiomyoma’s) and GIST’s. In the earlier series, GIST’s were described as leiomyomas, before they were recognized as a discrete entity. Taken together, leiomyoma’s and GIST’s together made up 53% of all SML’s in 13 EUS series[6] . In the esophagus, stomach, and duodenum they made up 77%, 54%, and 17% of all SML’s respectively[8]. It is now known that true leiomyomas are the most common type in the esophagus, while GIST’s are rare. In the stomach and small bowel GIST’s are the most common, and leiomyomas are rare. The common and rare SML’s in the esophagus, stomach, and duodenum are listed in Table 1.

Polkowski et al estimated that 13% of all pathologically verified SML’s in 22 case series including 826 patients were malignant, and another 8% were potentially malignant. The risk of malignancy in the esophagus is negligible (1%). The risk of malignancy in the stomach and duodenum are 16% and 5%, respectively. A further 6% of gastric and 19% of duodenal lesions are classified as potentially malignant, mainly including GIST’s of indeterminate malignant otential and carcinoid tumors[8].
EUS examination technique for SML
EUS examination with standard radial and linear echoendoscopes is best performed by water submersion method. The lumen is filled with de-aerated water after suctioning out all air and bubbles. The transducer balloon should be minimally inflated to avoid compression of the wall layers. The transducer must be kept at an optimal distance from the lesion, and tangential imaging should be avoided.
EUS examination technique for SML
EUS examination with standard radial and linear echoendoscopes is best performed by water submersion method. The lumen is filled with de-aerated water after suctioning out all air and bubbles. The transducer balloon should be minimally inflated to avoid compression of the wall layers. The transducer must be kept at an optimal distance from the lesion, and tangential imaging should be avoided.
Using conventional imaging frequencies, the gastrointestinal wall is displayed as 5 layers of alternating black and white echo-layers by endoscopic ultrasound (Figure 3). This layered structure on EUS remains the same throughout the gastrointestinal tract from the esophagus to the rectum. By convention, these are numbered from the inner-most (1st layer) to outer-most (5th layer). The 1st layer that appears bright (hyper-echoic) is due to the interface echo of the mucosa and surrounding fluid. This corresponds to the superficial mucosa. The 2nd (dark, hypoechoic) layer corresponds to the deep mucosa. The 3rd (hyperechoic) layer corresponds to the submucosa and the acoustic interface between the submucosa and the muscularis propria. The 4th (hypoechoic) layer represents the muscularis propria minus the acoustic interface. The 5th layer corresponds to the serosa and the sub-serosal fat, or the adventitia. When imaging with higher frequency probes at 20 MHz or 30 MHz esophageal wall can be visualized a 9-layer structure.

Complete EUS characterization of a SML includes: lesion diameter, layer of origin, echo-architecture (internal structure, echogenicity, texture, and any defined internal structures), margins, covering wall layers, internal perfusion, and ancillary findings like adjacent lymphadenopathy (Tables 2 and 3). The layer of origin can be determined by evaluating the ‘transition zone’ or the ‘junctional point’ of the SML with the normal gastrointestinal wall (Figure 4). However, a recent study indicated that only 79.6% of the tumors were correctly assigned to their layer of origin by EUS[9].
EUS guided tissue acquisition from SML
The presumptive diagnosis on EUS imaging alone and the final pathologic diagnosis match in 43% to 75% of cases[9,10]. EUS guided tissue sampling should be strongly considered for a hypoechoic lesion located in the 3rd or 4th ultrasonic layer, because malignant or potentially malignant lesions are included in the differential diagnosis[11]. In a recent study of EUS-guided sampling of 4th layer lesions, the diagnostic yield was 83.9%[12]. If the lesion is symptomatic, large in size, or has EUS features that are suspicious for malignancy, then a tissue diagnosis may not be essential, as surgery is anyway indicated. The need to obtain tissue from small (<1 cm) hypoechoic gastric wall masses is controversial. However, the American Gastroenterological Association (AGA), recommends that a tissue diagnosis should be considered in small gastric SML’s, given their potential for malignant behavior[11].



EUS sampling can provide cytological material, as well as tissue cores for histology. Histological material has been acquired by both standard EUS aspiration as well as by use of EUS-trucut biopsy needles (Figures 5A,5B,5C,5D,5E).
Histology sections show cells within their intact tissue stroma, while cytology smears allow resolution of microarchitecture and details of the nucleus and cytoplasm. EUSFNA with a modified sampling technique makes it possible to obtain a core tissue specimen from SML’s, which can be then examined histologically and immunohistochemically. Ando et al used a standard 22 G FNA needle to obtain core tissue specimens from mesenchymal tumors[36]. Other Japanese groups have reported similar results. Either the entire aspirate, or alternating drops of the aspirate are expelled in buffered 10% formalin solution, paraffin embedded, and thin sliced. For SML’s, a 22 G FNA needle is enough to obtain a tissue core as preservation of tissue architecture is not important, and the aim of obtaining a tissue core is immunohistochemical staining.
Two types of smears can be prepared from FNA material: air-dried and alcohol-fixed. Air-dried smears are stained rapidly (using a modified Romanowsky stain, like Diff-Quik TM) and used for immediate cytological evaluation. Diff- uik TM stained, air-dried smear preparations highlight intracytoplasmic material and extracellular substances. Alcohol fixation (95% ethyl alcohol) causes cells to shrink and round up, but preserves nuclear features. Alcohol fixed smears are stained by Papanicolau (Pap) or Hematoxylin-Eosin (H&E) stains. The Pap stain highlights nuclear detail. Pap stain and Diff-Quik TM stains are complementary, and optimal cytological information is obtained when both alcohol- ixed and air-dried smears are prepared from the FNA material. Alcohol-fixed smears, cyto-centrifuged preparations, thin- Prep TM preparations, or cell-block may be used for immunocytochemical stains. Some liquid fixatives contain methanol- a coagulative fixative (rather than a protein crosslinking fixative such as formalin), which may lead to suboptimal fixation for immunocytochemistry. Immunocytochemical staining on cytology smears has excellent correlation with immunohistochemical staining on core tissue specimens. In all cases requiring EUS-FNA, at least 1 separate pass should be submitted into cell-block for immunochemistry. However, cytology samples seldom contain more than 2 to 3 cell types, and this reduces the possibility of having an internal control. Hence negative immunocytochemistry results in cytological specimens are not as meaningful as positive reactions, and the true nature of a negative immunocytochemistry results is difficult to verify.


For flow cytometric analysis or molecular genetic analysis, the cells should be collected in RPMI-1640 solution or Hank’s balanced salt solution. RPMI-1640 is a cell culture transport medium used to preserve cells until they are made into a cell-block or send for flow cytometry. Collection of samples in Hank’s balanced salt solution is associated with the rapid loss of cell viability over a 24-hour period, which may decrease the yield of diagnostic cells for flow cytometry[13].
Cellular features of GIST’s can be seen in FNA samples (Figure 6). The average diagnostic yield of EUS-FNA for smooth muscle tumors (leiomyoma’s) and GIST’s was 82% in 10 reported series[8]. The yield of EUS-FNA cytology for the diagnosis of SML is less than other targets. The overall sensitivity of EUS-FNA for all malignant diseases is reported to be 80%-85%, with a positive predictive value of 100%, and an accuracy of 80%-90%. In contrast, the accuracy of EUSFNA for SML is only 50%, with almost half of the biopsy specimens being inadequate[14,15].
EUS assisted endoscopic resection of SML
Before endoscopic removal of any SML it is essential to characterize the layer of origin and rule out local invasion by EUS evaluation. EUS can also provide information about the vascularity of the lesion. Lesions confined to the mucosal or submucosal layers can be safely removed, although deeper lesions have also been endoscopically removed. A preprocedure tissue diagnosis of the SML is essential. EUS has also been used to inject saline precisely beneath deepseated SML’s, in order to improve the safety of endoscopic resection[16]. Submucosal injection of saline may cause the mucosal surface to level off near the tumor margins, if it is not placed directly beneath the tumor. In rare instances of deeply extending SML, the injection should be carried out under EUS guidance to separate the tissues. However, most authorities consider extension of the tumor to the muscle layer as a contraindication to endoscopic resection, because of a higher risk of perforation.
The technical options for endoscopic removal of an SML are standard snare polypectomy with or without submucosal saline injection, endoscopic mucosal resection with a ligating device (EMR-L), endoscopic mucosal resection with a transparent cap (EMR-C), unroofing in cases of lipomas or lymphangiomas, endoscopic enucleation using a snare or cutting knife, and endoscopic submucosal dissection (ESD) using an insulated -tip knife. Sun et al have described banding of esophageal, gastric, and duodenal leiomyomas of size < 15 mm, arising from the muscularis propria. They used a 10 mm cap to ligate a total of 64 leiomyomas, and allowed the lesions to slough spontaneously. The serosa or adventitia outside of the band gradually approximated in response to the local inflammation. No perforation or other complications occurred[17].
Well demarcated, hypoechoic lesions, upto 2 cm in size, which are limited to the muscularis mucosa or submucosa on EUS, can be removed by using standard snare polypectomy, EMR-L, or EMR-C methods. Hypoechoic and well - emarcated SML’s that are large in size (>2 cm) can be removed by endoscopic enucleation using a snare, a cutting knife, or an insulated-tip electrosurgical knife. Interested readers are referred to a recent review on the subject by Shim et al, providing excellent technical details[18].
EUS guided injection treatment of SML
A single case of ablation of a gastric GIST of size 4 cm by EUS-guided intra-tumoral injection of 95% ethanol has been described. A volume of 1.5 mL of alcohol was injected in a single session, using a 19-gauge FNA needle. There was no tumor recurrence at 2-years follow up[19].
Neoplastic lesions
The important neoplastic SML’s in the upper gastrointestinal tract are GIST, leiomyoma (and leiomyosarcoma), lipoma, carcinoid, granular cell tumor, schwannoma, glomus cell tumor, fibroma, neurofibroma, inflammatory myofibroblastic tumor, hemangioma, and mural metastasis. These lesions are described below.
Gastrointestinal stromal tumor (GIST)
GIST’s are the most common mesenchymal tumors of the gastrointestinal tract. These tumors originate from the interstitial cells of Cajal. Histologically, GIST’s can be characterized as spindle cell type (70%), epithelioid type (20%), or rare mixed type[20]. GIST’s of spindle cell type are composed of relatively uniform eosinophilic cells arranged in short fascicles or whorls. The tumor cells have paler eosinophilic cytoplasm than smooth muscle tumor cells. GIST’s of epithelioid type are composed of rounded cells with variably eosinophilic or clear cytoplasm, and uniform round-to- void nuclei with vesicular chromatin. Lesions of mixed cell type may exhibit an abrupt transition or complex mingling between spindle cell and epithelioid areas[21]. GIST’s can resemble smooth muscle tumors on light microscopy, but can be distinguished from them by immunocytochemical staining.
Most of the patients with GIST’s are between the ages of 40 to 80 years, with a median age at diagnosis of 60 years[22]. The majority of GIST’s occur in the stomach (60% to 70%) and small intestine (25% to 35%), with rare occurrence in the colon and rectum (5%), esophagus (<2%) and appendix. Some GIST’s are primary in the omentum, mesentery or retroperitoneum, and are unrelated to the tubular gastrointestinal tract. Most GIST’s are sporadic, but approximately 5% of GIST’s are associated with one of 3 tumor syndromes: neurofibromatosis type 1 (NF-1)[23], Carney’s triad (gastric GIST’s, paraganglioma, and pulmonary chondroma)[24], and familial GIST syndrome[25]. In NF-1 patients, GIST’s have a predilection for the small intestine, are often multiple, and the majority are clinically indolent. Approximately 3% of GIST’s are found in the pediatric population[26]. Children are more likely to have multifocal gastric tumors, harbor epithelioid histology, contain wild-type c-kit gene, and have higher rate of lymph node metastasis Gain-of -function mutations in c-kit can be detected in upto 85% of GIST’s, while another 3% to 5% of GIST’s harbor platelet derived growth factor -a (PDGFR- a) mutations. Most of the mutations are located in the juxtamembrane domain (exon 11) of the gene. Less commonly, mutations have been detected in the extracellular domain (exon 9), and tyrosine kinase domains (exons 13 and 17)[27]. Normally, the c-kit protein (CD 117) is a transmembrane receptor for a growth factor named stem cell factor, and has internal tyrosine kinase activity. Gain-of-function mutations in the c-kit gene in GIST, lead to ligand-independent dimerization, constitutional activation of the c-kit tyrosine kinase activity, and uncontrolled cell proliferation[28].
The c-kit protein is a highly sensitive and specific marker that differentiates GIST’s from other mesenchymal tumors like leiomyomas, schwannomas, or leiomyosarcomas. Kit positivity in GIST’s is strong, diffuse, and pan-cytoplasmic (even though kit is a transmembrane protein). The best kit antibodies currently available for formalin-fixed and paraffinembedded tissues are polyclonal ones, whereas most monoclonal kit antibodies show inconsistent reaction. Specific kit antibodies should react with normal kit-positive tissue components such as mast cells and interstitial cells of Cajal. Data on PDGFR- a expression in GIST’s is scant, and many commercially available antibodies are not reliable on paraffin embedded tissues[29]. Additional diagnostic criteria for GIST’s include common positivity for CD 34 (70%), vimentin (100%), variable expression of smooth muscle actins (20% to 30%) and S-100 protein (10%) and almostuniform negativity for desmin (only 2% to 4% of GIST’s are positive) and glial fibrillary protein.





On EUS, GIST’s appears as a hypoechoic tumor arising from the 4th sonographic layer or the muscularis propria. GIST’s can also arise from the 2nd sonographic layer (muscularis mucosa), and reside within the 3rd layer. GIST’s can be fast-growing tumors and can quickly outgrow their blood supply. As a result they can develop central necrosis, which is evident on EUS as central anechoic areas. The necrotic areas can fistulize to the gastrointestinal lumen and result in gastrointestinal bleeding[20]. The surface ulceration is seen on EUS as a localized break of mucosal layer (Figures 7A,7B,7C). Extramural component of a GIST may not be evident from its endoscopic appearance, and can be evaluated by EUS (Figures 8A,8B).
The EUS appearance of GIST’s is not specific and can be seen with other mesenchymal tumors, like leiomyomas and schwannomas. Heterotopic pancreas of the fusion type involves multiple gastric wall layers including the 4th, and can also mimic a GIST. Gastrointestinal autonomic nerve tumors (GANT’s) have a similar histological pattern, with epithelioid and spindle cells, but on electron microscopy they show features of autonomic neurons, with neurosecretory dense core granules and skeinoid fibers. GANT’s are now understood to be a variant of GIST, based on its similarity with GIST by histology, kit positivity, and kit mutations, and are not considered as a separate entity[30].
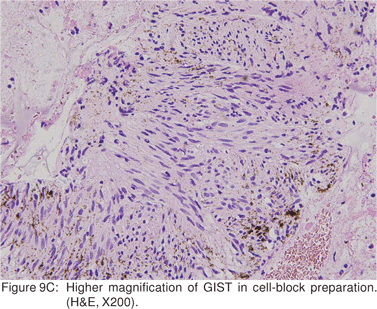
Endoscopic biopsies of GIST are frequently nondiagnostic, except in the setting of an ulcerated mass. Hence tissue sampling of a suspected GIST by EUS-FNA is essential to establish the diagnosis. EUS-FNA is the preferred method to biopsy suspected GIST’s. Percutaneous biopsy should not be used to confirm the diagnosis of GIST, as it can precipitate tumor rupture and lead to tumor dissemination or hemorrhage[20]. FNA samples from GIST’s show hypercellular groups of spindle cells and rarely epithelioid cells. The spindle cells show blunt-ended nuclei and may show nuclear angulations[13]. The major pitfall with EUS-FNA of GIST’s is the aspiration of muscle cells from the wall of the gastrointestinal tract. A panel of immunocytochemical stains including antibodies against c-kit (CD117), CD 34, SMA, muscle specific actin, and S-100 must be used (Figures 9A,9B,9C,9D). Hunt et al retrospectively compared the morphological features of GIST’s with CD 117 negative spindle cell tumors. Compared to the latter, GIST’s more often displayed a size of > 40 mm, cystic spaces, and mucosal ulceration. The specificity of these criteria for diagnosis of GIST was as high as 92%, but the sensitivity was only 65%[31].
Approximately 10%-30% of all GIST’s display malignant behavior. The frequency of malignant GIST’s is 20% to 30% of all soft-tissue sarcomas[27], but small benign tumors, often found incidentally during unrelated surgery or autopsy, are probably much more common.
Various studies have evaluated different EUS morphologic criteria to predict the risk of malignancy in GIST’s. Most of the studies are retrospective, and most were conducted before GIST’s were differentiated from smooth muscle tumors. In an individual patient the behavior of GIST may be difficult to predict unless the tumor metastasizes or recurs after resection. The endosonographic features suggesting a higher risk of malignancy in GIST’s are[32,33,34 ,35,36]:
· Tumor size > 30 mm to 40 mm
· Irregular margins
· Echogenic foci
· Cystic spaces
· Heterogeneous echotexture
· Exophytic development
· Non-round or non-oval shape
· Adjacent lymphadenopathy
· Ulcerated mucosa

Traditional cytological features of malignancy like hyperchromasia, nuclear polymorphism, or increased nuclear/cytoplasmic ratios are not consistently seen in malignant GIST’s. Mitotic figures are seldom seen in FNA smears, and therefore mitotic count that is used to define the malignant potential of a GIST is not possible from cytology smears. However, mitotic counts can be performed on trucut biopsy samples from GIST’s. Mitotic activity and tumor size are independent predictors of malignancy in a GIST (Table 4). The mitotic activity of GIST’s is typically expressed per 50 hpf (X 40), totaling an area of 5 mm[2]. If modern widefield microscopes with wide-field eyepieces are used, the mitotic count should be adjusted to correspond to the same total area. This would equal approximately 25 fields at X 40 magnification[29].


Cellular proliferation estimated by immunocytochemical markers of cell proliferation (Ki-67, MIB-1, and PCNA) may be independent prognostic factors, but have not been studied in detail[36,37]. Ki-67 positive cells can be easily recognized in EUS-FNA cytology specimens[36]. Ki-67 monoclonal antibody identifies a large nuclear phosphoprotein of unknown function. Ki-67 protein is expressed in all nuclei except those in G0 phase, thus being a marker for cells that have entered the cell cycle and potentially proceeding into mitotic division. The percent of Ki-67 positive nuclei is referred to as the Ki-67 index or proliferation index (Figures 10A,10B,10C,10D)[38]. MIB-1 is a monoclonal antibody analog of Ki-67 that can be applicable to formalin fixed tissues. Analysis of c-kit gene mutation is also feasible on both cytological and histological biopsy specimens.[39] Hence EUS-FNA can be used to confirm the diagnosis of a GIST, but do not provide definite information about the lesion’s malignant potential.




Because of the unpredictable biological behavior of GIST’s, and the suboptimal predictive value of currently available tissue sampling methods, definite terms such as benign or malignant GIST are best avoided. EUS features should be considered as a broad guide of the risk of malignancy. The AGA technical review on gastric SML’s, recommends a cut-off size of 3 cm for deciding whether surgical resection is indicated for an asymptomatic GIST[11]. For small intestinal GIST’s, which have a higher malignant potential, tumor size may not be a reliable indicator of malignancy. There is currently no consensus regarding the optimal management strategy for small, asymptomatic GIST’s. Monitoring of tumor size is not a reliable way to detect malignancy, as both malignant and benign tumors may grow in size. Conversely, tumors that do not seem to grow on follow up cannot be assumed to be benign, and even a small, mitotically inactive tumor can occasionally metastasize[8]. Hence every GIST should be considered as potentially malignant, or precancerous lesions8. Expectant management for GIST’s can be pursued in patients who are asymptomatic, have small tumors (<2cm), or are poor surgical candidates.
Leiomyoma and leiomyosarcoma
Leiomyoma’s comprise of bundles of interlacing smooth muscle cells, and are well demarcated from adjacent tissue by a connective tissue capsule. The tumor cells are spindle shaped, with cigar shaped nuclei. They usually do not show increased mitoses, but focal minimal cytological atypia may be seen.
Leiomyoma’s are most commonly found in the distal third of the esophagus, but can also be found in the rectum and colon. In the esophagus, leiomyomas outnumber GIST’s by a ratio of 3:1. Majority of esophageal leiomyoma’s are asymptomatic and discovered incidentally. Leiomyoma’s are benign in the vast majority of cases[40], although leiomyosarcomas have been reported. Esophageal leiomyoma’s rarely cause symptoms when they are smaller than 5 cm in diameter. If a leiomyoma is suspected at esophagoscopy, biopsies should not be performed, as inflammation and scarring at the biopsy site would hamper extramucosal resection or enucleation at surgery[41]. On EUS, leiomyoma’s appear as round or oval shaped, hypoechoic, homogenous tumors, arising from the 2nd or 4th sonographic wall layers. In a large recent study, 62.1 % of esophageal leiomyoma’s were found to originate from the muscularis mucosa[42]. This EUS appearance is indistinguishable from GIST’s and other mesenchymal tumors. Very small leiomyoma’s may be mistaken for intramural cysts, as they may appear almost anechoic. Occasionally, leiomyomas may contain calcifications. Leiomyoma’s and leiomyosarcomas should be differentiated from GIST’s by positive immunoreactivity for desmin and actin and negative immunoreactivity for c-kit (CD117) and CD34 (Figures 11A,11B,11C).
Asymptomatic leiomyoma’s with typical EUS features do not require treatment. Surgical excision is recommended for symptomatic leiomyomas and those greater than 5 cm in size. Minimally invasive open surgical or thoracoscopic enucleation of esophageal leiomyoma’s is feasible and safe (Figures 12A,12B,12C,12D).





Lipoma
Lipomas originate from the submucosa and appear as round, well-circumscribed tumors with a homogeneous yellow surface on cross section. Lipomas consist of mature adipocytes (Figure 13A). If surface ulceration is present, inflammation with fat necrosis, fatty cysts, and foamy macrophages may be seen. Rare reports of malignant transformation to liposarcoma have been reported.
Endoscopically, lipomas are seen as a compressible, smooth bulge with a yellowish hue. A ‘pillow sign’ or ‘cushion sign’ is seen as a persisting indentation after pushing the closed biopsy forceps into the tumor. The pillow sign has 98% specificity but only 40% sensitivity for identifying lipomas[10]. On EUS, lipomas appear as hyperechoic, homogenous tumors located in the submucosa (Figures 13B). This appearance is diagnostic for lipomas[43]. Incidentally found lipomas do not require treatment.
Carcinoid
Grossly, carcinoids appear as smooth, rounded, yellow nodules. Larger tumors may be irregularly shaped with depression or ulceration, often associated with fibrosis. Carcinoid tumors originate from enterochromaffin - like cells in the mucosa and may involve deeper layers, especially the submucosa. The round or polygonal tumor cells, with centrally located round nuclei, are arranged in ribbon or trabecular patterns and may form rosettes. The cytoplasm appears faintly eosinophilic or coarsely granular and eosinophilic (Figure 14A,14B). In the stomach carcinoid tumors can be classified as: Type-1 associated with hypergastrinemia and chronic atrophic gastritis, type-2 associated with Zollinger- Ellison syndrome and multiple endocrine neoplasia type 1 (MEN-1), and type-3 which are sporadic tumors. Type-1 and - 2 carcinoids are usually multiple and rarely become locally invasive or metastatic. Type-3 gastric carcinoids display malignant behavior in upto half of patients, and require resection[11]. Endoscopically, carcinoid tumors appear as round, polypoid lesions, with or without central depression or a bridging fold. They usually have normal overlying mucosa and seldom ulcerate (Figure 15). Most carcinoid tumors can be diagnosed by forceps biopsy or by polypectomy excision.
EUS examination shows a hypoechoic, homogenous or inhomogeneous (‘pepper-and-salt’ appearance), well marginated lesion originating from the deep mucosa or submucosa (2nd of 3rd sonographic layer) (Figures 16,17A,17B,17C)[44]. Other neuroendocrine tumors of thegastrointestinal tract share this EUS appearance. Small (<1cm) gastric and duodenal carcinoids that do not penetrate the muscularis propria can be treated by endoscopic excision. Carcinoid tumors of >1cm in size have a 10% of lymph node metastasis, and require an informed decision for their management. Carcinoids that invade the muscularis propria require surgical resection, irrespective of their size[45]. After endoscopic resection, close follow up is recommended including repeat EUS examinations.
Granular cell tumor (GCT; Synonyms: Granular myoblastoma, Abrikossoff tumor)
Abrikossoff first described the histological features of this tumor in 1926. These rare tumors are most commonly located in the tongue (40%), followed by skin and subcutaneous tissues (30%), breast (15%), respiratory tract (10%), and gastrointestinal tract (6%), and other rare locations. Of all gastrointestinal GCT’s, around one-third (2% overall) are found in the esophagus[46]. In the esophagus, most GCT’s are found in the distal part. In a large study of 110 patients, GCT’s were found in the lower esophagus in 65%-75%, inthe mid esophagus in 18%-20%, and in the upper esophagus in 5%-15% of cases[47]. Esophageal GCT’s can be multiple in approximately 10% of cases[48]. In the stomach, no site predilection is seen.
Grossly granular cell tumors are firm, pale yellow nodules, which usually do not exceed 2 cm in diameter, originating from the deep mucosa or submucosa. Microscopically, GCT’s are composed of nest to sheets of round-to-polygonal cells, with abundant eosinophilic granular cytoplasm. These cytoplasmic granules are PAS-positive and diastase resistant. Multinucleated cells, nuclear polymorphism, and mitotic activity are rare. The origin of GCT’s is from Schwann cells, and the tumor cells show positive immunoreactivity for S- 100 protein, vimentin, neuron specific enolase, and nestin, and are negative for desmin, actin, CD 34 and c-kit.
Clinically most GCT’s are detected incidentally and are benign. The malignancy rate in these tumors is estimated to be less than 2%[49]. Associated tumors have been reported, but it is not clear whether they were responsible for the endoscopic investigation themselves.
Endoscopically, GCT’s appear as white to yellowish nodules with smooth, non-ulcerated surface (Figure 18A). Endoscopic biopsies can be diagnostic in upto 50% of cases (Figure 18B)[51]. On EUS, they appear hypoechoic, mildly inhomogeneous, well-defined lesions arising from the 2nd or 3rd sonographic layers (deep mucosa or submucosa) (Figure 18C)[50,51]. Atypical EUS findings may predict the rare malignant GCT. There is a single case report of endoscopic injection of polidocanol to achieve necrosis in a GCT in a 53 year old woman[52]. For asymptomatic GCT’s that are not excised, surveillance EUS every 1-2 years is recommended to monitor change in size. (Book-Fockens) Local endoscopic snare excision can be performed for small tumors.
Schwannoma
Schwannoma’s, also known as neurilemmomas, are slowgrowing neoplasms originating in any nerve that has a Schwann cell sheath. Schwannoma’s grossly resemble GIST’s. They are tan to white, well circumscribed, spherical or ovoid shaped, small (<5 cm) intramural spindle cell tumors. They mostly originate from the muscularis propria and may show a fibrous capsule. Schwannoma’s consist of spindle cells with nuclear palisading, and often form a microtrabecular pattern intermingled with fibrovascular septa (Figure 19). These tumors have a low mitotic activity of < 5/50 hpf. The tumor cells are positive for S-100 protein and glial fibrillary acidic protein, and non-reactive for CD 34, CD 117, actin, HHF-35, desmin, melan-A and HMB-45.
Gastrointestinal schwannoma’s usually occur in the stomach (60%-70%) or colon (20%-30%) in older individuals[29]. A few cases have been described in the esophagus[53]. In the stomach they represent 0.2% of all tumors[54]. The EUS appearance of schwannoma’s is similar to any mesenchymal tumor. It is important that they be distinguished from GIST’s by their immunocytochemical staining, because even if they are large, schwannoma’s are usually benign[55,56].
Glomus cell tumors
Glomus cell tumors originate from modified vascular smooth cells. They usually occur in the peripheral soft tissues but can also occur in the gastrointestinal tract, almost exclusively the In stomach (Figure 20A)57. These tumors grossly appear as red-blue nodules originating in the muscularis propria. The tumor consists of small, uniform, round glomus cells that are located in the walls of dilated vascular spaces (Figure 20B). The tumor cells have small, uniform nuclei, show positive immunoreactivity for actin, and are negative for c-kit, desmin, and S-100 protein . Hyperplastic smooth muscle cells often surround the lesion. Glomus cell tumors are usually benign.
EUS shows a hypoechoic, well-circumscribed tumor located in the 3rd and or 4th sonographic layer (Figures 20C). However EUS morphologic findings are insufficient for diagnosis and EUS-FNA is required[58].
Fibroma and neurofibroma
These rare mesenchymal tumors are found in the submucosal layer. They are less hyperechoic than lipomas. There are no distinctive EUS features. Neurofibromas are positive for S-100 protein, and negative for actin on immunocytochemistry[58].
Inflammatory myofibroblastic tumor (IMFT)
These tumors consist of proliferating myofibroblasts and a mixed inflammatory infiltrate composed of lymphocytes, eosinophils, histiocytes, and plasma cells, within a collagenous or myxoid matrix (Figure 21A). The cellular composition is more heterogeneous than a GIST. The tumor cells are c-kit and CD34 negative, but can be actin positive (Figure 21B)[58]. The etiology may be an exaggeratedproliferative response to infection, trauma, peptic ulcer, surgery, radiotherapy, chemotherapy, or steroid use[59].
IMFT are most common in the lung, but can arise in any organ. They have been reported to occur in the esophagus, stomach, small intestine, colon, appendix, pancreas, liver, and spleen. IMFT can simulate a GIST, especially when forming an intramural mass in the gastrointestinal tract. These tumors usually occur in children and young adults. They are usually benign, but can grow rapidly and be locally invasive. They can present with epigastric pain, weight loss, anemia, or fever of unknown origin.
IMFT originate from the submucosa, and endoscopic biopsies are usually negative. On EUS, they appear as hypoechoic, well-defined lesions in the submucosa[60].
Hemangioma
Hemangiomas are rare tumors of the esophagus. In a review of 19,982 autopsies, esophageal hemangiomas were found in 3 patients[61]. Hemangiomas of the esophagus may present with dysphagia and bleeding, or may be asymptomatic. They may be located in any part of the esophagus. Hemangiomas may appear as sessile or pedunculated polyps covered by normal mucosa, with or without a bluish or reddish hue. They may be mistaken for esophageal varices. On EUS, hemangiomas appear as a well-defined hypoechoic mass arising from the 2nd or 3rd sonographic layers[62,63].This appearance is not characteristic. Successful endoscopic resection of these lesions has been described without bleeding complication.
Metastasis, primary carcinomas, and adenomas
Endoscopic appearance of gastric metastasis can resemble either a submucosal tumor (51%) or primary gastric cancer (39%). The final diagnosis can be obtained by endoscopic biopsies in over 90% cases, and EUS-FNA is usually not needed[64]. The most common primary sites for metastasis to the stomach are malignant melanoma, breast, lung, and esophagus. Rarely, adenocarcinoma of the stomach can assume a polypoid appearance, and can resemble an ulcerated GIST endoscopically (Figure 22). Very rarely, the surface of a predominantly subepithelial extending adenocarcinoma may remains intact, and the tumor can appear as a SML (Figures 23A,23B,23C). Adenomas are uncommon in the stomach, but can become large in the duodenum and be endoscopically difficult to characterize. EUS shows their superficial origin, and can rule out deep mural invasion before endoscopic resection (Figures 24A,24B).
Non-neoplastic lesions
Non-neoplastic SML’s of the upper gastrointestinal tract are Brunner’s gland hamartoma, aberrant pancreas, inflammatory fibroid polyps, fibrovascular polyps, submucosal cysts, and varices. These lesions are described below.
Brunner’s gland hamartoma (Brunneroma)
Brunner’s gland hamartoma account for approximately 5% of all benign duodenal tumors, and is found in 0.008% of autopsy cases[65]. Histologically, Brunner’s gland hamartoma is composed of submucosal proliferation of normal Brunner’s glands, stromal proliferation, and various combinations of adipose tissue, lymphoid aggregates, and stromal fibrosis (Figure 25).
Clinically, these lesions can present with acute or chronic gastrointestinal bleeding, or duodenal obstruction[66]. Endoscopically, they appear as broad based, sessile or pedunculated tumors, in the first or second part of duodenum. The sizes of these lesions usually range from 1 cm to 6 cm in diameter. The surface of the lesion is smooth or may show small ulcers. They can transiently regress and enlarge with antisecretory treatment and H. pylori eradication[67]. Endoscopic biopsy is usually non-diagnostic. On EUS, Brunner’s gland hamartoma appear as a hypoechoic, heterogeneous mass, with small cystic spaces, in the deep mucosa and submucosa (2nd and 3rd sonographic layers). Multiple large cystic spaces can also be seen inside the lesion[68]. The margins of the lesion are indistinct because of proliferation of Brunner’s glands, lymphoid follicles, and fibrous tissue[66].
Aberrant pancreas
Aberrant pancreas is defined as pancreatic tissue that has neither anatomic nor vascular continuity with the normally located pancreas. Its prevalence in autopsy series has been estimated to be between 0.6%-13.7%[68]. In 90% of cases, aberrant pancreatic tissue is found in the stomach, duodenum, or proximal part of the jejunum. Aberrant pancreas appears macroscopically as solitary, hemispheric, umbilicated mass measuring up to 4.0 cm in diameter. Aberrant pancreas is usually located in the submucosa, but may extend to the muscularis propria and subserosa. Aberrant pancreas displays the characteristics of orthotopic pancreas, containing pancreatic acini and ducts, and occasional islets, and hypertrophic smooth muscle fibers. Aberrant pancreas has been be classified by Heinrich into 3 histological types: type I includes all elements of normal pancreatic tissue, type II includes pancreatic tissue without islet cells, and type III includes pancreatic ducts only. If smooth muscle and duct-like structures are the only components, as in Heinrich type III, the lesion can be described as an adenomyoma. Secondary changes such as pancreatitis, cysts, or neoplasia may be seen, although the majority of aberrant pancreas are asymptomatic.



Endoscopically, aberrant pancreas appears as a submucosal nodule with a central umbilication or depression, which represents a draining pancreatic duct (Figures 26A). Histological diagnosis is difficult with standard endoscopic biopsies[69]. On EUS, aberrant pancreas appears hypoechoic relative to the surrounding submucosa, with a heterogeneous echotexture. The margins of the lesion are usually indistinct because of the lobular structure of acinar tissue at the margin (Figure 26B)[70]. If the acinar tissue is densely gathered, the margins will be better defined on EUS. Aberrant pancreas is located within both of submucosa and muscularis propria (fusion type), or within the submucosa alone (separate type)[70]. ‘Fusion type’ aberrant pancreas is usually Heinrich type I, while separate type is Heinrich types II or III. Thickening or hypertrophy of the muscularis propria (4th layer thickening) is usually seen, even when the ectopic pancreatic tissue does not extend directly into this layer. The cause of this muscular layer thickening is not clear. Small, scattered hyperechoic areas in the lesion represent adipose tissue. Small internal anechoic areas correspond to ductal structures[70]. Tissue sampling to establish the diagnosis is essential, as the differential diagnosis of hypoechoic 3rd layer lesions include potentially malignant lesions (Figures 26C)[11]. No pancreatitis has been reported after FNA of aberrant pancreas in the literature. Asymptomatic lesions do not require treatment.
Inflammatory fibroid polyps
Esophageal or gastric inflammatory fibroid polyps (IFP) are submucosal fibroblastic, inflammatory lesions, typically forming ulcerated intraluminal polyps. These rare, benign lesions are composed of non-encapsulated fibrous tissue, many small blood vessels, and an eosinophilic infiltrate. They are c-kit negative, but may be positive for CD 34. On EUS, IFP are located in the 2nd and 3rd sonographic layers (deep mucosal or submucosal layers) without involvement of the muscularis propria[71,72,73,74]. They are typically hypoechoic, homogenous, and have indistinct margins. Sometimes, slightly hyperechoic appearance can be seen because of multiple small vessels within the fibrous stroma of the polyps
Fibrovascular polyps
Fibrovascular polyps (FVP) are found in the cervical esophagus, and extend into the esophageal lumen for a variable distance. FVP are extensions of the lamina propria and are composed of fibrous, vascular, and adipose tissue. FVP are covered by normal squamous epithelium[75]. EUS evaluation of FVP is difficult, but in one report, the lesion was described to originate from the submucosa[76].
Submucosal cystic lesions
Submucosal cystic lesions can be vascular or non-vascular. Vascular submucosal cysts include varices and hemangiomas. Anechoic, non-vascular (cystic) subepithelial lesions include: heterotopic gastric glands, cystic lymphangiomas (lymphatic cysts), heterotopic pancreas, simple cysts, Brunner’s gland hamartoma, mucocele, mural abscess, and myogenic tumor or gastric tuberculoma with extensive cystic degeneration[77]. Morphologically, cystic SML can be divided into simple cystic, polycystic or septated, and solid-cystic lesions. Simple cystic lesions are duplication cysts, heterotopic gastric mucosa, or rarely Brunner’s gland hamartoma. Multicystic lesions can be lymphangiomas, heterotopic gastric mucosa, or Brunner’s gland hamartoma. Solid-cystic SML includes heterotopic pancreas, lymphangiomas, tuberculoma, or GIST with cystic degeneration.
Varices: EUS can detect fundal varices earlier and more often than standard endoscopy. Varices appear as anechoic structures in the submucosa. They can be traced as tubular structures, and perigastric vessels can be detected.
Duplication cysts: Foregut duplication cysts can be of enterogenous (50%-70%) or bronchogenic (7%-15%) subtype[78]. Foregut cysts are classified by their histological features rather than by location. Bronchogenic cysts are lined by respiratory (ciliated pseudostratified columnar) epithelium (Figures 27A), and may contain cartilage and bronchial glands in their wall. Enterogenous cysts are lined by alimentary (squamous or enteric) epithelium (Figure 27B)[79]. Upto 50% -60% of these cysts may contain gastric mucosa or pancreatic tissue80. A cyst that does not contain cartilage, has a double layer of surrounding smooth muscle, and has attachment or close proximity to the esophagus is probably esophageal in origin. Bronchogenic cysts are located in the mediastinum around the tracheobronchial tree or within the pulmonary parenchyma. The walls of duplication cysts can contain one or more layers of smooth muscle.
Although duplication cysts are most prevalent in the pediatric age group, upto 30% of duplication cysts may be diagnosed after the age of 12 years[81]. Duplication cysts occur most commonly in the ileum, esophagus, and colon, and may be contained within the wall of the gastrointestinal tract or may be extrinsic to it. Around 10% to 15% of all gastrointestinal duplications are esophageal, and may be attached to the esophagus in a paraesophageal location or may be intramural[82]. Most esophageal duplication cysts are located in the right posterior inferior mediastinum. Nearly two-thirds of duplication cysts are located in relation to the lower third, and one third each in upper and middle third of esophagus[82]. Gastric duplications are rare, and the majority are found in females. Usually gastric duplication cysts occur along the greater curvature of the stomach and share a common wall and blood supply (Figures 28A,28B)[83]. Intramural cysts can also rarely occur as a result of resolved inflammatory process.


On CT scan the cysts have water density contents, but cysts containing mucoid material are often misdiagnosed on CT scans as solid masses or lymph nodes. Barium swallow examination may demonstrate a smooth, rounded impression on the esophagus. Radionuclide scans using 99m Tc sodium pertechnetate may be helpful in children, in whom upto 50% of thoracic duplication cysts contain ectopic gastric mucosa. Bronchogenic cysts are non-enhancing, may contain cartilage in their wall, and if they communicate with the tracheobronchial tree, an air-fluid level may be seen[79].
Endoscopy shows a submucosal bulge with normal overlying mucosa. Endoscopic biopsies should be avoided because they do not provide useful information, and may complicate subsequent surgical excision of these cysts. On EUS, duplication cysts are seen to arise from the 3rd sonographic (submucosal) layer, or may be extrinsic to the gastrointestinal wall. The walls of duplication cysts may be seen as 3- or 5 layer pattern. However, this wall layer pattern may not always be seen around the cyst. The internal contents may be completely anechoic or hypoechoic Figures 29A,29B,29C,29D)[84]. The endosonographer should take the time to observe peristalsis in a juxta-enteric cyst. Peristalsis in a duplication cyst appears as ring contractions, with the entire cyst wall contracting concentrically inwards. As a result, the configuration of the cyst can be seen to change. Peristalsis in a juxta-enteric cyst is specific for a duplication cyst and should be considered as a diagnostic feature (Figures 30A,30B,30C)[85]. The cysts may contain thick mucinous material, septations, fluid levels, or debris. The abundant mucinous aspirate has lead to misdiagnosis of a gastric duplication cyst as a mucinous neoplasm[86]. FNA reveals cell debris, hemosiderin-laden macrophages, as well as ciliated columnar cells and goblet cells in bronchogenic cysts, and squamous cells in esophageal duplication cysts[87]. Detached ciliary tufts in the FNA aspirate of duplication cysts have also been described[88]. However aspiration of duplication cysts should not be routine, and should only be undertaken with caution under antibiotic cover. Infection of the cysts after EUS-FNA with mediastinal abscess formation has been reported[89].







Malignant degeneration of foregut cysts is rare[90]. However, the surgical literature suggests that a large proportion of foregut cyst may develop complications[81]. Hence, optimal management of asymptomatic patients with duplication cysts is still controversial.
Gastritis cystica profunda or heterotopic gastric glands are characterized by elongation of gastric foveolae, and hyperplasia and cystic dilatation of the gastric glands. These dilated glands extend through the muscularis mucosae into the submucosal layer. In most cases, gastritis cystic profunda develops in patients who have undergone gastroenterostomy, with or without gastric resection. In these cases, the lesion usually appears on the gastric side of the anastomosis. Uncommonly, it can also occur in an unoperated stomach[91]. Gastritis cystica profunda can present as giant gastric folds, as a SML, or as an isolated polyp[92]. It is considered to be a precancerous lesion. EUS shows multiple anechoic areas, or a polycystic lesion with hyperechoic septae, located in the thickened 3rd sonographic (submucosal) layer.
Lymphangiomas are usually found in the small and large intestine, and rarely in the stomach or esophagus. They are benign lesions that consist of dilated lymphatic vessels situated in the submucosal or mucosal layers. An epithelial layer covers their inner wall, and they are subdivided by irregular septal structures with smooth muscle or connective tissue. On endoscopy, they appear as easily compressible, broad based, polypoid lesions. On EUS, lymphangiomas may appear as multiple anechoic structures, with hyperechoic septae in the 3rd sonographic (submucosal) layer. The lesion can also appear as simple or septated anechoic lesions. If the lymphatic vessels are very small, the overall echogenicity may appear as inhomogeneous or hypoechoic.
Algorithm for evaluation of SML (Figure 31)
The first step is to distinguish between extramural and intramural lesions. Lesions in the first two (1st and 2nd sonographic layers) layers can be diagnosed by endoscopic biopsies, or can be removed by standard endoscopic methods. For all other non-vascular lesions arising from deeper layers, the presence or absence of symptoms, and the EUS morphology of the lesion dictates whether the lesion needs resection or EUS-guided sampling. If the patient is symptomatic (bleeding, mass effect, or obstruction), then FNA is not necessary and surgical resection should be considered regardless of the lesion size. Similarly, if the lesion morphology is suspicious for malignancy, then the result of FNA results will not change management regardless of the results.

Hyperechoic, homogenous 3rd layer lesions are always lipoma’s, and need no further evaluation. Dark 3rd layer lesions can include both benign and potentially malignant lesions. After including vascular lesions, stacked (tunneled) biopsies can be used to obtain tissue diagnosis. The drawback of taking biopsies from 3rd and 4th layer lesions with intact overlying mucosa for subsequent surgical enucleation has been discussed. 3rd layer lesions can also be resected completely by endoscopic submucosaldissection (ESD). For diagnosis of 4th layer lesions, EUSFNA with immunochemical stains may be used, if the result can be expected to change the management, as discussed previously. C-kit positive spindle cell tumors should be resected or closely followed up. C-kit negative, SMA+, or S100 positive tumors define leiomyomas or schwannomas, which do not need resection. An algorithmic approach to SML’s of the upper gastrointestinal tract based on the available evidence is presented in Figure 31.
1. Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc.1991;5:20–3.
2. Takubo K, Nakagawa H, Tsuchiya S, Mitomo Y, Sasajima K, Shirota A. Seedling leiomyoma of the esophagus and esophagogastric junction zone. Hum Pathol.1981;12:1006–10.
3. Abraham SC, Krasinskas AM, Hofstetter WL, Swisher SG, Wu TT. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol. 2007;31:1629–35.
4. Motoo Y, Okai T, Ohta H, Satomura Y, Watanabe H, Yamakawa O, et al. Endoscopic ultrasonography in the diagnosis of extraluminal compressions mimicking gastric submucosal tumors. Endoscopy. 1994;26:239–42. Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, et al; German EUS Club. Endoscopic ultrasonography. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856–62.
5. Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33–54.
6. Kim EU. Submucosal lesions. In: Endosonography. Eds: Hawes RH and Fockens P. Saunders Elsevier. 2006. China.pp:99–110.
7. Polkowski M. Endoscopic ultrasound and endoscopic ultrasoundguided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–45.
8. Polkowski M. Endoscopic ultrasound and endoscopic ultrasoundguided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–45.
9. Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–8.
10. Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–8.
11. Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218–23.
12. Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218–23.
13. Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound- guided fine-needle aspiration. A cytopathologist’s perspective. Am J Clin Pathol . 2003;120:351–67.
14. Wiech T, Walch A, Werner M. Histopathological classification of non neoplastic and neoplastic gastrointestinal submucosal lesions. Endoscopy. 2005;37:630–4.
15. Sun S, Wang M, Sun S. Use of endoscopic ultrasound-guided injection in endoscopic resection of solid submucosal tumors. Endoscopy. 2002;34:82–5.
16. Sun S, Wang M, Sun S. Use of endoscopic ultrasound-guided injection in endoscopic resection of solid submucosal tumors. Endoscopy. 2002;34:82–5.
17. Shim CS, Jung IS. Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy. 2005;37:646–54.
18. Shim CS, Jung IS. Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy. 2005;37:646–54.
19. Chaudhry UI, DeMatteo RP. Management of resectable gastrointestinal stromal tumor. Hematol Oncol Clin North Am. 2009;23:79–96.
20. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–65.
21. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–8.
22. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–8.
23. Carney JA. The triad of gastric epithelioid leiomyosarcoma, functioning extra-adrenal paraganglioma, and pulmonary chondroma. Cancer. 1979;43:374–82.
24. Carney JA. The triad of gastric epithelioid leiomyosarcoma, functioning extra-adrenal paraganglioma, and pulmonary chondroma. Cancer. 1979;43:374–82.
25. Agaram NP, Laquaglia MP, Ustun B, Guo T, Wong GC, Socci ND, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204–15.
26. Miettinen M, Majidi M, Lasota J.Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38:S39–51.
27. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80.
28. Miettinen M, Lasota J. M. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78.
29. Miettinen M, Lasota J. M. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–78.
30. Hunt GC, Rader AE, Faigel DO. A comparison of EUS features between CD-117 positive GI stromal tumors and CD-117 negative GI spindle cell tumors. Gastrointest Endosc. 2003;57:469–74.
31. Chak A, Canto MI, Rösch T, Dittler HJ, Hawes RH, Tio TL, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468–73.
32. Chak A, Canto MI, Rösch T, Dittler HJ, Hawes RH, Tio TL, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468–73.
33. Brand B, Oesterhelweg L, Binmoeller KF, Sriram PV, Bohnacker S, Seewald S, et al. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis. 2002;34:290–7.
34. Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, et al; German EUS Club. Endoscopic ultrasonography. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856–62.
35. Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43.
36. Okubo K, Yamao K, Nakamura T, Tajika M, Sawaki A, Hara K, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004;39:747–53.
37. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83.
38. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83.
39. Mutrie CJ, Donahue DM, Wain JC, Wright CD, Gaissert HA, Grillo HC, et al. Esophageal leiomyoma: a 40-year experience. Ann Thorac Surg. 2005;79:1122–5.
40. Punpale A, Rangole A, Bhambhani N, Karimundackal G, Desai N, de Souza A, et al. Leiomyoma of esophagus. Ann Thorac Cardiovasc Surg. 2007;13:78–81.
41. Xu GQ, Zhang BL, Li YM, Chen LH, Ji F, Chen WX, et al. Diagnostic value of endoscopic ultrasonography for gastrointestinal leiomyoma.World J Gastroenterol. 2003;9:2088–91.
42. Yasuda K, Cho E, Nakajima M, Kawai K. Diagnosis of submucosal lesions of the upper gastrointestinal tract by endoscopic ultrasonography. Gastrointest Endosc. 1990;36:S17–20.
43. Matsumoto T, Iida M, Suekane H, Tominaga M, Yao T, Fujishima M. Endoscopic ultrasonography in rectal carcinoid tumors: contribution to selection of therapy. Gastrointest Endosc. 1991;37:539–42.
44. Yoshikane H, Tsukamoto Y, Niwa Y, Goto H, Hase S, Mizutani K, et al. Carcinoid tumors of the gastrointestinal tract: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1993;39:375–83.
45. Yoshikane H, Tsukamoto Y, Niwa Y, Goto H, Hase S, Mizutani K et al. Carcinoid tumors of the gastrointestinal tract: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1993;39:375–83.
46. Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301–16.
47. Perçinel S, Sava B, Yilmaz G, Erinanç H, Küpana Ayva S, Bektaº M, Ensari A.Granular cell tumor of the esophagus: three case reports and review of the literature. Turk J Gastroenterol. 2008;19:184–8.
48. Ordóñez NG. Granular cell tumor: a review and update. Adv Anat Pathol. 1999;6:186–203.
49. Palazzo L, Landi B, Cellier C, Roseau G, Chaussade S, Couturier D, Barbier J. Endosonographic features of esophageal granular cell tumors. Endoscopy. 1997;29:850–3.
50. Love MH, Glaser M, Edmunds SE, Mendelson RM. Granular cell tumor of the oesophagus: endoscopic ultrasound appearances. Australas Radiol. 1999;43:253–5.
51. Maccarini MR, Michieletti G,Tampieri I, Triossi O, Bertinelli E, Casetti T. Simple endoscopic treatment of a granular-cell tumor of the esophagus. Endoscopy. 1996;28:730–1.
52. Murase K, Hino A, Ozeki Y, Karagiri Y, Onitsuka A, Sugie S. Malignant schwannoma of the esophagus with lymph node metastasis: literature review of schwannoma of the esophagus. J Gastroenterol. 2001;36:772–7.
53. Murase K, Hino A, Ozeki Y, Karagiri Y, Onitsuka A, Sugie S. Malignant schwannoma of the esophagus with lymph node metastasis: literature review of schwannoma of the esophagus. J Gastroenterol. 2001;36:772–7. Stelow EB, Lai R, Bardales RH, Linzie BM, Mallery S, Stanley MW. Endoscopic ultrasound-guided fine-needle aspiration cytology of peripheral nerve-sheath tumors. Diagn Cytopathol . 2004;30:172–7.
54. Stelow EB, Murad FM, Debol SM, Stanley MW, Bardales RH, Lai R, et al. A limited immunocytochemical panel for the distinction of subepithelial gastrointestinal mesenchymal neoplasms sampled by endoscopic ultrasound-guided fine-needle aspiration. Am J Clin Pathol. 2008;129:219–25.
55. Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301–11.
56. Matsui M, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumors. Endoscopy. 1998;30:750–5.
57. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–72.
58. Shah SM, Sussman D, Jorda M, Ribeiro A. EUS with EMR of an inflammatory myofibroblastic tumor of the stomach. Gastrointest Endosc. 2008;67:561–3.
59. Plachta A. Benign tumors of the esophagus. Review of literature and report of 99 cases. Am J Gastroenterol. 1962;38:639–52.
60. Araki K, Ohno S, Egashira A, Saeki H, Kawaguchi H, Ikeda Y, et al. Esophageal hemangioma: a case report and review of the literature. Hepatogastroenterology. 1999;46:3148–54.
61. Yoshikane H, Suzuki T, Yoshioka N, Ogawa Y, Ochi T, Hasegawa N. Hemangioma of the esophagus: endosonographic imaging and endoscopic resection. Endoscopy. 1995;27:267–9.
62. Oda, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33:507–10.
63. Hizawa K, Iwai K, Esaki M, Suekane H, Inuzuka S, Matsumoto T, et al. Endosonographic features of Brunner’s gland hamartomas which were subsequently resected endoscopically. Endoscopy. 2002;34:956–8.
64. Block KP, Frick TJ, Warner TF. Gastrointestinal bleeding from a Brunner’s gland hamartoma: characterization by endoscopy, computed tomography, and endoscopic ultrasound. Am J Gastroenterol. 2000;95:1581–3.
65. Weisselberg B, Melzer E, Liokumovich P, Kurnik D, Koller M, Bar- Meir S. The endoscopic ultrasonographic appearance of Brunner’s gland hamartoma. Gastrointest Endosc. 1997;46:176–8.
66. Armstrong CP, King PM, Dixon JM, Macleod IB. The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg. 1981;68:384–7.
67. Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric aberrant pancreas: EUS analysis in comparison with the histology. Gastrointest Endosc. 1999;49:493–7.
68. Hase S, Nakazawa S, Yoshino J, Kojima Y, Niwa Y, Ohashi S, et al. A study on gastric and small intestinal aberrant pancreas by endoscopic ultrasonography—with special reference to comparison with histological appearance. Nippon Shokakibyo Gakkai Zasshi. 1989;86:1684–91.(article in Japanese)
69. Matsushita M, Uchida K, Nishio A, Okazaki K. Endoscopic and EUS features of gastric inflammatory fibroid polyps. Gastrointest Endosc. 2009;69:188
70. Matsushita M, Takakuwa H, Nishio A. Characteristic endosonographic features of gastric inflammatory fibroid polyps. Endoscopy. 2001;33:729–30.
71. Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric inflammatory fibroid polyps: endoscopic ultrasonographic analysis in comparison with the histology. Gastrointest Endosc. 1997;46:53–7.
72. Matsushita M, Okazaki K. Characteristic endoscopic features of gastric inflammatory fibroid polyps. J Gastroenterol Hepatol. 2005;20:1310.
73. Schuhmacher C, Becker K, Dittler HJ, Höfler H, Siewert JR, Stein HJ. Fibrovascular esophageal polyp as a diagnostic challenge. Dis Esophagus. 2000;13:324–7.
74. Devereaux BM, LeBlanc JK, Kesler K, Brooks J, Lehman GA, Sherman S, Ciaccia D. Giant fibrovascular polyp of the esophagus. Endoscopy. 2003;35:970–2.
75. Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712–4.
76. Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005;128:2893–909.
77. Reed JC, Sobonya RE. Morphologic analysis of foregut cysts in the thorax. Am J Roentgenol Radium Ther Nucl Med. 1974;120:851–60.
78. Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest. 1997;112:1344–57. Whitaker JA, Deffenbaugh LD, Cooke AR. Esophageal duplication cyst. Case report. Am J Gastroenterol. 1980;73:329–32.
79. Whitaker JA, Deffenbaugh LD, Cooke AR. Esophageal duplication cyst. Case report. Am J Gastroenterol. 1980;73:329– 32.
80. Anderson MC, Silberman WW, Shields TW. Duplications of the alimentary tract in the adult. Arch Surg. 1962;85:94–108.
81. Simstein NL. Congenital gastric anomalies. Am Surg. 1986;52:264–8.
82. Geller A, Wang KK, DiMagno EP. Diagnosis of foregut duplication cysts by endoscopic ultrasonography. Gastroenterology. 1995;109:838–42.
83. Bhatia V, Garg PK, Gupta SD, Dash NR, Saluja SS, Madan K. Demonstration of peristalsis in gastric duplication cyst by EUS: implications for diagnosis and symptomatology. Gastrointest Endosc. 2008;68:183–5.
84. Wang B, Hunter WJ, Bin-Sagheer S, Bewtra C. Rare potential pitfall in endoscopic ultrasound-guided fine needle aspiration biopsy in gastric duplication cyst: a case report. Acta Cytol. 2009;53:219–22.
85. Jhala N, Jhala D. Gastrointestinal tract cytology: advancing horizons. Adv Anat Pathol. 2003;10:261–77.
86. Eloubeidi MA, Cohn M, Cerfolio RJ, Chhieng DC, Jhala N, Jhala D, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of foregut duplication cysts: the value of demonstrating detached ciliary tufts in cyst fluid. Cancer . 2004;102:253–8.
87. Wildi SM, Hoda RS, Fickling W, Schmulewitz N, Varadarajulu S, Roberts SS, et al. Diagnosis of benign cysts of the mediastinum: the role and risks of EUS and FNA. Gastrointest Endosc. 2003;58:362–8.
88. Kuraoka K, Nakayama H, Kagawa T, Ichikawa T, Yasui W. Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: a case report with literature review. J Clin Pathol. 2004;57:428–31.
89. Bechade D, Desrame J, Algayres JP. Gastritis cystica profunda in a patient with no history of gastric surgery. Endoscopy. 2007;39:E80–E81.
90. Park JS, Myung SJ, Jung HY, Yang SK, Hong WS, Kim JH, et al. Endoscopic treatment of gastritis cystica polyposa found in an unoperated stomach. Gastrointest Endosc. 2001;54:101–3.
|
|
|
 |
|
|